- Acquired Intestinal Obstruction
Acquired Intestinal Obstruction
Introduction
Bowel obstruction (or intestinal obstruction) is a mechanical or functional obstruction of the intestines, preventing the normal transit of the products of digestion.
Intestinal obstruction can occur at any age from newborn infants to adults. The etiology of the obstruction varies greatly, depending on the age that it occurs and the past surgical history.
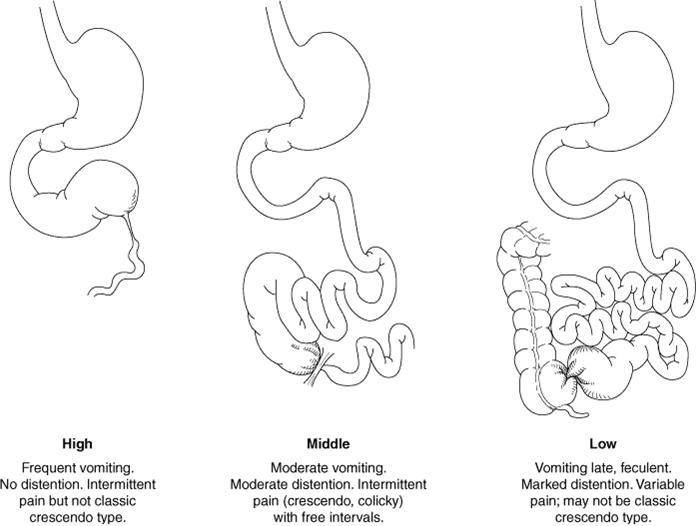
The lesions that cause intestinal obstruction can be separated into high, middle, low anatomic obstructions, and functional obstructions.
|
|
|
Image 1. Types of anatomic bowel obstructions |
High anatomic obstructions are caused by lesions that interrupt bowel continuity proximal to the midportion of the jejunum. Low anatomic obstructions are distal to the midportion of the jejunum. Functional obstructions may be caused by sepsis, electrolyte imbalance, necrotizing enterocolitis and hypothyroidism.
Small bowel obstruction (SBO) in infants and children is more common than large bowel obstruction. There are many causes, both congenital and acquired, including adhesive small bowel obstruction; hernias, which may be congenital or acquired; and intramural and extramural intestinal lesions. By far the most common of these is adhesive small bowel obstruction, which accounts for up to 60% of small bowel obstructions. Adhesions are followed by tumors (20%), hernias (10%), inflammatory bowel disease (5%), volvulus (3%), and various miscellaneous causes of intestinal obstruction [43].
Ileus (or functional obstructions) is a disruption of the normal propulsive ability of the gastrointestinal tract. Although ileus originally referred to any lack of digestive propulsion, including any bowel obstruction, up-to-date medical usage restricts its meaning to those disruptions caused by the failure of peristalsis, rather than by mechanical obstruction.
Classification of Intestinal Obstruction
|
|
- paralytic (adynamic) ileus - spastic ileus
- strangulated intestinal obstruction - obturation intestinal obstruction - mixed intestinal obstruction |
|
|
|
NB: Bowel obstruction is the syndrome that occurs in many diseases, leading to disruptions in the passage of chyme.
Acquired Mechanical Intestinal Obstruction Causes
The most common causes of bowel obstruction in children:
1) Intussusceptions; 2) Adhesions
The less common causes of bowel obstruction in children:
1) Volvulus; 2) Herniations; 3) Foreign body; 4) Tumors; 5) Diverticulitis.
|
Symptoms of bowel obstraction |
|
|
|
Diagnosis |
- Plan X-ray - Contrast X-ray (barium sulfate)
|
|
|
|
|
|
|
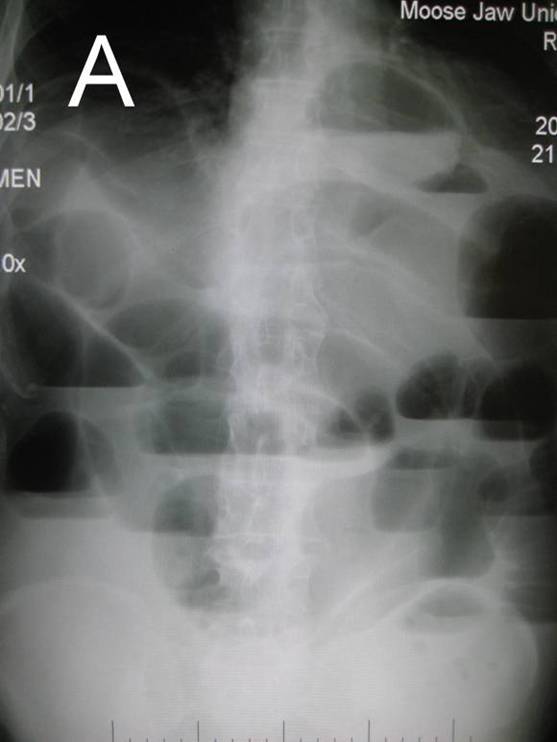
Image 2. Plan abdominal X-ray in upright position. The main roentgenological sign of bowel obstruction: A. Air-fluid levels; B. Dilated loops of bowel. |
||
Intussusception
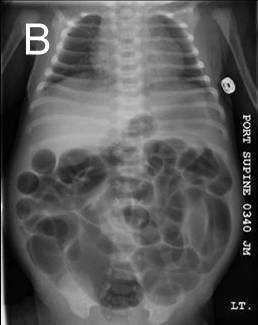
Intussusception, the telescoping or invagination of a proximal portion of intestine (intussusceptum) into a more distal portion (intussuscipiens), is one of the most common causes of bowel obstruction in infants and toddlers.
Vascular compromise and subsequent bowel necrosis are the primary concerns with intussusception. In addition to bowel obstruction, edema with venous obstruction and eventual obstruction of arterial flow leads to ischemia and eventual full-thickness necrosis of the intussuscepted bowel and mesentery.
Although uncommon, in patients who undergo operative reduction of intussusception, as many as 10% may require bowel resection [14].
|
|
Image 3. Illustration of an intussusception is showing the invaginated intussusceptum (blue) and the invaginating intussuscipiens (red). A - demonstrates a direct or normograde intussusception occurring in the direction of normal peristalsis (most common type). B - demonstrates an indirect or retrograde intussusception occurring against the normal direction of peristalsis. |
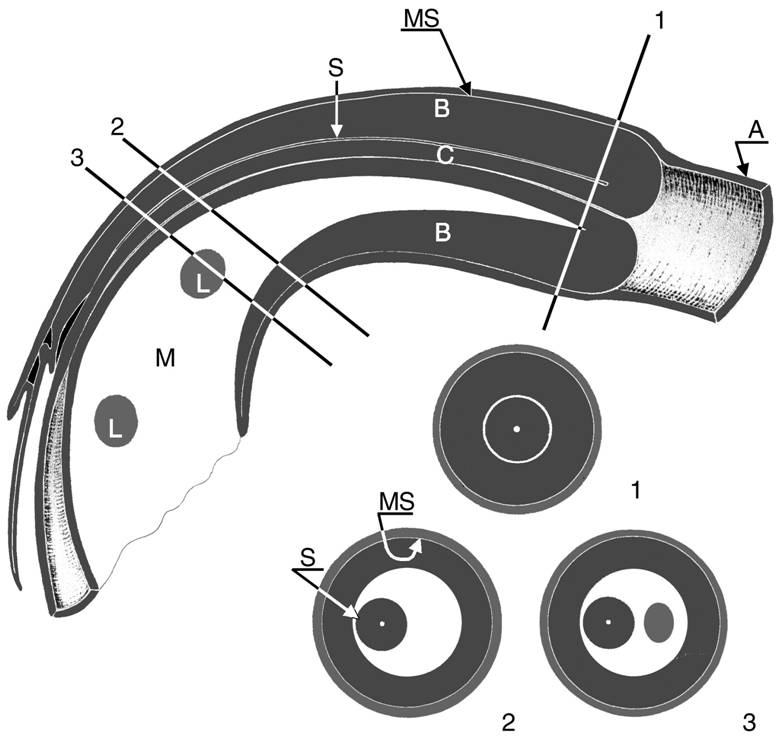
Image 4. Longitudinal view and three axial views of an intussusception; three bowel loops and the mesentery can be seen. The intussuscipiens (A) contains the two limbs of the intussusceptum: the everted returning limb (B), which is edematous, and the central entering limb (C), which is located at the center of the intussusception with the accompanying mesentery (M). The mesentery contains some lymph nodes (L). MS = contacting mucosal surfaces of the intussuscipiens and everted limb, S = contacting serosal surfaces of the everted limb and central limb.
History
Intussusception was first described by Barbette in 1674, and Wilson was the first to successfully treat it surgically in 1831. In 1876, Hirschsprung first reported the technique of hydrostatic reduction, and after monitoring a series of 107 cases, reported a 35% mortality rate in 1905 [45].
Frequency
Intussusception primarily affects infants and toddlers, although it can also occur prenatally or during the neonatal period. Intussusception rarely occurs in adults.
The estimated incidence is about 1.5-4 cases per every 1,000 live births. Males are affected more than females at a ratio of 3:2 [6, 14, 36].
Intussusception is primarily a disorder of infancy and occurs most commonly between 5–10 months of age [2, 16]. Two thirds of children with intussusception are less than 1 year of age at presentation.
Incidence peaks during two seasons of the year: spring/summer and middle of winter. This seasonal variation correlates with times of increased number of cases of viral gastroenteritis and upper respiratory infection [7, 43].
Pathogenesis
The pathogenesis of intussusception has been described to an inhomogeneity of longitudinal forces along the intestinal wall.
In the resting state, normal propulsive forces meet a certain resistance at any point. This stable equilibrium can be disrupted when a portion of the intestine does not appropriately promulgate peristaltic waves.
Small perturbations provided by contraction of the circular muscle perpendicular to the axis of longitudinal tension result in a kink in the abnormal portion of the intestine, creating a rotary force (torque). Distortion may continue, in-folding the area of inhomogeneity and eventually capturing the circumference of the small intestine. This invaginated intestine then acts as the apex of the intussusceptum.
Intramural, intraluminal, or extramural processes may produce points of disequilibrium. Along with anatomic abnormalities, flaccid areas that follow a paralytic ileus can also create unstable segments because adjoining areas create discordant contractions with the return of bowel activity.
Pathophysiology
Intussusception results in bowel obstruction, followed by congestion and edema with venous and lymphatic obstruction. This progresses to arterial obstruction and subsequent necrosis of the bowel. Ischemia and then necrosis results in fluid sequestration and bleeding from the GI tract. If untreated, the bowel may perforate and the patient becomes septic, peritonitis can lead to death.
Etiology
Intussusception is most commonly idiopathic and no anatomic lead point can be identified. The vast majority of cases are idiopathic (95%) [14, 16, 36].
An anatomic lead point that draws the proximal intestine and its mesentery inward and propagates it distally through peristalsis is identified in only 5% of cases and is most commonly found in cases of ileoileal intussusception.
Anatomic lead points are more commonly found in children older than 1 year and almost always in adults with intussusception.
The most commonly encountered anatomic lead point is a Meckel’s diverticulum.
Several viral gastrointestinal pathogens (adenovirus, rotavirus, reovirus, echovirus) may cause hypertrophy of the Peyer’s patches (mural lymphoid tissues) of the terminal ileum which may potentiate bowel intussusception [36, 45].
Intussusception may sometimes occur as a complication of some medical conditions, including:
- · Viral infections (especially adenovirus)
- · Meckel's diverticulum
- · Intestinal polyps
- · Tumors, such as lymphosarcoma and neurofibroma
- · Lymphoma
- · Cystic fibrosis
- · Recent abdominal surgery
- · Henoch-Schonlein purpura
- · Inflammatory bowel disease
- · Hemophilia
- · Hemangioma
Children with cystic fibrosis (CF) may present with intussusception due to inspissated meconium in the terminal ileum. While generally observed as a complication in older children with CF, neonatal intussusception with meconium plug syndrome associated with CF has been reported [14].
Classification
(by H. Feldman, 1977)
|
|
The most common form is ileo-colic in 80-90% of cases, less often ileo-ileal occurs in up to 15% and rarely caeco-colic, jejuno-jejunal or even ileo-ileo-colic occur in a double or three-fold manner [2, 5, 6, 8].
Clinical presentation
The initial symptoms may include:
- Abdominal pain (83%) – usually severe and comes on suddenly, colicky or cramping; in children, this may be indicated by drawing knees to chest and crying.
- Stools mixed with mucus and blood (often described as currant jelly) (53%) – in 5-6 hours from onset [3].
- Palpable abdominal mass
This classic triad is present in only one third of infants with intussusception.
Additional symptoms include:
- Food refusal
- Vomiting (sometimes yellow or green tinged)
- Dehydration
- Fever
The infant with intussusception has a history of severe cramping or colicky abdominal pain occurring intermittently every 5-30 minutes. During these attacks, the infant screams and flexes at the waist, draws the legs up to the abdomen, and become pale and diaphoretic. These episodes may last for only a few seconds and are separated by periods of calm normal appearance and activity.
Between attacks, the infant may appear somnolent or quite normal, and findings on examination of the abdomen may be quite unremarkable.
Upon initial inspection, the abdomen may appear scaphoid; during paroxysms, it may be rigid; and later in the course of the illness, it may become distended with signs of peritonitis.
Careful palpation after an attack has subsided may reveal “sausage-shaped” mass. With early ileocolic intussusception, the mass is typically found in the right upper quadrant or abdomen. The right lower quadrant may seem empty upon examination, a finding known as the Dance sign [1, 6, 8, 36]. This mass may be difficult to locate in inconsolable infants because of abdominal rigidity from muscle straining.
Early on, vomiting of undigested food may occur. As attacks continue, emesis may turn bilious.
Stools mixed with mucus and blood often described as currant jelly, a sign of intestinal ischemia and mucosal sloughing.
The rectal examination should commence with inspection of fecal material in the diaper. Normal-appearing stool should be tested for occult blood. The presence of mucoid or frankly bloody stool supports the diagnosis.
Rarely, inspection of the anus may reveal the prolapsed tip of the intussusception. Prolapse of the intussusceptum from the anus is a rare event (1-3%). A digital rectal examination should be performed routinely, looking for blood or a mass higher in the anal canal.
If the delay in diagnosis allows bowel ischemia to occur, subfebrile fever, tachycardia, and hypotension can be signs of bacteremia and bowel perforation.
The babies with intussusception are usually well nourished and are generally above average in physical development. This fat and healthy appearance is apt to mislead the physician if he or she sees the baby in the early hours of illness.
Diagnosis
Tests include:
- Blood and urine tests
- Fecal occult blood test, which checks the stool for blood
- Abdominal x-ray
- Ultrasound or CT scan
- Diagnostic and therapeutic enema (Air contrast or water-soluble contrast enema)
Blood chemistry abnormalities are not specific for intussusception [2, 4, 8, 14, 16]. Depending on the duration of illness and associated vomiting and blood loss, laboratory investigations may reflect dehydration, anemia, leukocytosis, or a combination of these.
Early in the course of the illness, findings on plain radiographic examination of the abdomen (supine and upright) may show a normal or nonspecific bowel gas pattern. Later, findings suggestive of intussusception include dilated loops of small bowel with or without air-fluid levels, an airless or opacified right lower quadrant, or both. In 25-60%, abdominal plain films demonstrate a right upper quadrant soft tissue density that displaces air-filled loops of bowel. Occasionally, the intussusceptum may be apparent on plain abdominal radiography.
Ultrasonography of the abdomen is a reliable means to identify intussusception. Two ultrasonographic signs of intussusception are: the “doughnut” or “target” sign on transverse views, and the “pseudokidney” sign on longitudinal views [5, 16, 36].
The evaluation of abdominal pain often leads to CT examination. Although not indicated for the diagnosis of intussusception, intussusception can be found incidentally on CT scan [14, 43].
Differentiation
Disorders characterized by bowel obstruction, colicky abdominal pain, blood in the stool, an intra-abdominal mass, or a combination of these should be considered in the differential diagnosis of intussusception. These include gastroenteritis, appendicitis, Meckel diverticulum, malrotation with midgut volvulus, rectal mucosal prolapse, polyps, Henoch-Schonlein purpura or incarcerated hernia.
Diagnostic Procedures:
Diagnostic and therapeutic enema.
Air contrast or water-soluble contrast (e.g. Triombraste/Verografin) enema is the “gold-standard” diagnostic study for infants with suspected intussuception. It is both diagnostic and therapeutic in identifying and reducing intussusception. The diagnostic enema is therapeutic in 80-90% of patients [14, 16, 36, 43].
NB: Pneumatic enema or hydrostatic enema is used to confirm the diagnosis and to reduce the intussusception.
The pneumatic reduction technique under fluoroscopy has gained wide acceptance because of several advantages over hydrostatic reduction: it is easy to perform and can be done quickly, is less messy, delivers less radiation exposure, is more comfortable, and results in smaller perforations and less peritoneal contamination [43].
The incidence of perforation has been higher with pneumatic reduction and varies between 1% and 2,8% [14].
Treatment
Conservative treatment:
The use of contrast enemas allows direct visualization of the reduction under fluoroscopic control and is reported to be successful in 80% to 90% of cases [8, 14, 16, 36].
Conservative treatment (contrast enemas) is contraindicated if the [1, 14, 36, 43]:
- Recurrent intussusception
- Patient’s age > 1 year or < 3 month
- Appearance time of first symptoms more than 18 hours
- X-ray evidence of a ileo-ileal (small intestine) intussusception
- GI bleeding – “currant jelly” (time of stool mixed with mucus and blood more than 10 hours)
- If the child has signs of peritonitis
The pneumatic enema technique
A lubricated straight catheter is placed into the rectum and secured by taping the buttocks together tightly. While many radiologists prefer a balloon-tipped catheter, laceration or perforation of the rectum is a risk with balloon inflation.
A manometer and blood pressure cuff are connected to the catheter, and air is insufflated slowly to a pressure of 70-80 mm Hg (maximum 120 mm Hg) and followed fluoroscopically (or ultrasonographically) as it percolates proximally through the colon [14, 36, 43]. The column of air stops at the intussusception, and a plain radiograph is taken.
Each attempt should persist until reduction of the intussusception fails to progress for a period of 3-5 minutes. A maximum of three attempts should be made.
If no intussusception exists or if the reduction is successful, air (or other contrast) is observed to rapidly pass into the small bowel. Reflux of air into the terminal ileum, seen flouroscopically, signifies reduction of the intussusception.
If the intussusception is successfully reduced, an oral diet is resumed on the next morning.
If the intussusception cannot be completely reduced, operative intervention is indicated.
Surgical treatment
Preoperative details: Preoperatively, IV crystalloid resuscitation is begun (10 mL/kg x 2, plus 1.5 x maintenance fluid). A nasogastric tube is placed. Broad-spectrum IV antibiotics are administered. Body temperature must be preserved in the operating room. A type and screen of the patient's blood should be obtained.
The standard incision in infants is a fairly small right-sided transverse incision above or below the umbilicus [1].
After inspection for signs of perforation, the intussusception is identified and delivered into the wound. First, an attempt is made at manual reduction by retrograde milking of the intussusceptum (retrograde pressure is applied by squeezing the intussusceptum within the intussucipiens in a proximal direction).
Although gentle pulling may aid in reduction, avoid vigorous pulling a part of the intussuscepted segment of bowel. Following successful reduction, it is important to assess bowel viability and search for anatomic lead points.
After successful reduction, the previously intussuscepted bowel may look quite congested, bruised, and possibly not vital. The leading edge of the intussusceptum may look particularly ischemic. In almost all cases the bowel will become pink and vital after application of warm saline towels for less than 10-20 minutes [3, 8, 43].
An incidental appendectomy is often performed, particularly if a right lower quadrant incision was made for access to the abdomen, as it may be presumed that the patient has had an appendectomy [14].
If there is any doubt about the viability of the bowel after reduction, it should be resected. It is rarely feasible to resect or invert a small area of suspected necrosis.
In most patients, a primary end-to-end anastomosis can be fashioned after the ischemic bowel is resected. If the infant is in critical condition or unstable, the ischemic bowel can be quickly resected and both bowel ends exteriorized as temporary stomas [1, 3, 5, 8, 43].
Local or segmental resection is indicated if:
o the intussusception cannot be reduced,
o the segment of bowel appears infarcted or nonviable, or
o a lead point is identified.
Laparoscopy
Recent studies have reported successful laparoscopic reduction of intussusception in more than 60% of patients [43].
Most described laparoscopic techniques use three ports (one on the umbilicus and two on the left side of the abdomen).
Particular attention, however, must be paid to search for a pathologic lead point because most tactile cues are lost.
Complications
Complications after laparotomy and laparoscopy for intussusception include common postoperative problems such as wound infection, fascial dehiscence, and SBO.
Reported complications rates are lower (4%) when no enterotomy or bowel resection had to be performed (26%). The risk of postoperative adhesive SBO after operation for nonperforated intussusception compares with the rate for any pediatric laparotomy. Most cases (80%) occur within the first 2 years [43].
Outcomes
The recurrence rate of intussusception after successful reduction (whether hydrostatic or surgical) is about 5-20%. Recurrence may be slightly lower with reduction using air insufflation. The mortality rate of intussusception is less than 1% [14]. Mortality increases with delay in diagnosis, inadequate fluid resuscitation, perforation, and surgical complications.
Postoperative intussusception
In series from large children's hospitals, postoperative intussusception accounts for 1,5% to 6% of all cases of intussusception. The incidence of postoperative intussusception after laparotomy is 0,08% to 0,5%, but this process may complicate cardiac, thoracic, and orthopedic procedures [14, 45].
The cause of postoperative intussusception is believed to be altered peristalsis due to prolonged or excessive manipulation of the bowel, bruising of the intestine, anesthetic agents, or other neurogenic factors. The higher incidence of postoperative intussusception seen in children who have known dysmotility suggests that abnormal propulsion of the intestine may be an important factor. Lead points from anastomotic suture lines are rarely found.
The intussusception is most frequently located in the small intestine [16, 45].
Nonischemic (chronic) intussusception
About 15% of cases of intussusception in children may be described as subacute (symptoms of 4 to 14 days) or chronic (symptoms greater than 14 days) [45].
Patients with nonischemic intussusception present with recurrent mild to moderate abdominal discomfort and other nonspecific GI complaints, including vomiting, diarrhea, rectal bleeding, and failure to thrive. Ischemic compromise of the intussusception is rarely found, and abdominal masses are infrequently appreciated in this group.
This nonspecific presentation and frequently normal abdominal examination lead to the common but erroneous diagnosis of gastroenteritis. The presence of pink mucoid semiloose excrement may lead the examiner to suspect the diagnosis of chronic intussusception. Nonischemic intussusception should be included in the differential diagnosis of prolonged cases of vomiting and diarrhea, particularly if stools are positive for occult blood. Awareness of this entity will lead to correct diagnosis, and appropriate therapy can be initiated [45].
Neonatal intussusception
Neonatal intussusception, with symptoms occurring in the first 30 days of life, is rare (0,3% of all cases) [43].
60% to 75% of newborn infants with intussusception are found to have surgical lead points [45].
Signs and symptoms resemble those seen in necrotizing enterocolitis such as abdominal distension, bilious gastric aspirates, bloody stools, and rarely a palpable abdominal mass. Difficulties in establishing the correct diagnosis led to a delay of 7 to 10 days between the onset of symptoms and abdominal surgery, increasing the risk of developing a compromised bowel. Sometimes, the disconnected end of the intussusceptum can be found in the distal part of the bowel [43].
Diagnostic features are signs of SBO on the abdominal radiographs.
An early diagnosis may be achieved with a high index of suspicion and the use of ultrasound scan. Contrast enema has limited diagnostic and therapeutic capability [43].
Once the diagnosis of neonatal intussusception is confirmed, surgery is the preferred treatment option [6, 14]. There is a high incidence of surgical lead points, a low rate of successful enema reduction in small infants, and a greater risk of bowel perforation in infants younger than 6 months of age undergoing pressure reduction [45]. If diagnosed in time, it can be treated successfully with resection and primary anastomosis.
These cases carry a mortality of around 20% in neonates, largely because of sepsis and the delay in diagnosis [43].
Adhesive Intestinal Obstruction
Adhesion-induced obstruction is the most common cause of intestinal obstruction in general.
The incidence of postoperative small bowel obstruction in children ranges from 2% to 30% and is greater in neonates [43]. Obstruction occurred most often within 2 years of the initial operation (82%). 80% of obstructions occurred within 3 months of the initial operation, and 70% were secondary to a single adhesive band [6, 14, 16, 36, 43].
NB: If baby has abdominal pain and a history of any surgery on the abdominal organs, it is necessary first of all to keep in mind acute adhesive intestinal obstruction!
In children, the most common inciting operation was appendectomy, and there was no difference in occurrence after perforated, nonperforated, or negative appendectomies [43].
Procedures with the highest risk for future adhesive intestinal obstruction in pediatric patients include: colectomy, Ladd's procedure, nephrectomy, resection/reduction of intussusception, hepatectomy, Nissen fundoplication.
Adhesive small bowel obstruction most commonly develops after pelvic surgery including appendectomy, gynecologic procedures, and colorectal surgery. The obstruction is thought to be secondary to adhesive band formation in the pelvis, where the bowel is more mobile and likely to twist and obstruct around the adhesions.
Classification
Clinical types of adhesive intestinal obstruction [1]:
· Early type - onset of obstructive symptoms can occur within the first postoperative month.
· Late type - obstruction occur after the first postoperative month.
Etiology
Adhesions are fibrous bands of tissue that form between loops of bowel or between the bowel and the abdominal wall after intra-abdominal inflammation.
Obstruction occurs when the bowel is compressed or tethered due to these fibrous bands. This may result in kinking of the bowel, volvulus of a segment, or herniation of bowel between a band and another fixed structure within the abdomen.
Clinical Presentation
Children with mechanical intestinal obstruction present with colicky abdominal pain, distension and vomiting. In cases of prolonged intestinal obstruction, the vomitus may become bilious or even feculent [2, 4, 7, 8]. The child may be hemodynamically stable or may show signs of severe dehydration or sepsis (tachycardia, hypotension and fever).
Abdominal examination may reveal a distended abdomen with either hyperactive bowel sounds (obstruction) or a paucity of sounds (ileus). Patients may have obstipation depending on whether they have a complete or partial obstruction.
Differential diagnosis
The differential diagnosis is ileus versus mechanical obstruction.
Differentiating a prolonged postoperative ileus from postoperative bowel obstruction can be difficult. Radiographic demonstration of dilated bowel loops may not distinguish between the two entities [43].
Diagnosis
The key to the diagnosis of adhesive bowel obstruction is abdominal distention and bilious emesis in a patient with previous abdominal surgery.
In the early stages of intestinal obstruction, it may be difficult to discern obstruction from infectious gastroenteritis. Initially, the emesis may be nonbilious, but with time it progresses to bilious or "feculent" emesis. The child complains of crampy abdominal pain and has anorexia. With a partial obstruction there continues to be passage of flatus or small stools. In children with complete obstruction, both cease. As the obstruction progresses, the child becomes increasingly lethargic. The presence of a fever should make one suspect bowel compromise.
Physical findings may not be initially obvious, but abdominal distention with either high-pitched or hypoactive bowel sounds evolves over time. Eventual progression of the obstruction leads to continuous, localized pain that is not relieved by nasogastric decompression.
NB: Nonsurgical, inflammatory and metabolic conditions that may result in ileus must be considered.
Radiographs can help differentiate obstruction from infectious causes:
¤ Flat and upright abdominal films are obtained. Obstruction is manifested by dilated bowel loops with air-fluid levels. The presence of air in the colon and rectum may signify a partial bowel obstruction. Free intraperitoneal air is indicative of bowel perforation and requires urgent operative treatment.
¤ The diagnosis of intestinal obstruction can be confirmed with CT or a contrast-enhanced upper gastrointestinal series with small bowel follow-through (UGISBFT).
NB:
- Upper gastrointestinal series or upper GI series, also upper gastrointestinal tract radiography, is a radiologic examination of the upper gastrointestinal tract. It consists of a series of X-ray images of the esophagus, stomach and duodenum.
- Small-bowel follow-through, also called small-bowel series, is a radiologic examination of the small intestine from the distal duodenum/duodenojejunal junction to the ileocecal valve.
Patient drinks radiopaque contrast, most often barium sulfate (dose - 1/3 of one feeding age). X-ray images of abdomen are made at timed intervals (every 3-6 hours).
The advantage of a CT scan is that it can rule out other diagnoses, can identify the transition zone of the obstruction, and uses water-soluble contrast that does not become diluted as rapidly as the water-soluble contrast with UGISBFT in the presence of obstruction [43].
Treatment
Emergency surgery after preoperative preparations (2-4 hours) is considered only if there are symptoms of peritonitis, in other cases, treatment must be started from conservative measures [1, 3, 5, 8].
Conservative management includes resuscitation with isotonic saline solutions, nasogastric decompression, correction of electrolyte abnormalities, broad-spectrum antibiotics, and serial examinations.
Within 24-48 hours, children with ileus will improve as indicated by a return of bowel function, normalization of vital signs and normal white blood cell count.
Conservative treatment is successful in 70-80% of patients.
Indications for operation include obstipation, progressive or persistent abdominal tenderness, fever or leukocytosis despite conservative treatment and adequate resuscitation within 24-48 hours [14].
However, in Ukrainian medical school the permitted time for conservative treatment depends from a type of adhesive intestinal obstruction [1]:
- for early type – 8-12 hours
- for late type – 4-6 hours
The reason of this difference is a concept that late type of adhesive intestinal obstruction occur mostly due to strangulation of bowel by abdominal fibrous bands. Therefore more active surgical management can prevent the necrosis in the strangulated small bowel.
Surgery (open or laparoscopic) may only involve lysis of adhesive bands or it may necessitate bowel resection.
Postoperatively, nasogastric decompression and intravenous fluids with broad-spectrum antibiotics are continued until bowel function returns and the volume of gastric aspirate decreases to a minimum.
Prevention
A multitude of reports have described the prevention of intra-abdominal adhesions; however, a "magic bullet" to prevent adhesion formation has yet to be found [43].
General principles of gentle bowel handling, careful hemostasis, irrigation of the abdominal cavity, and prevention of prolonged bowel exposure to air have not eliminated the occurrence of adhesions [33].
Commercially available adhesion barriers such as Seprafilm, a hyaluronic acid and carboxymethylcellulose membrane, have been widely used and publicized. Several clinical trials using Seprafilm have demonstrated a decreased incidence of postoperative adhesions. However, one should avoid wrapping Seprafilm around an anastomosis because it may lead to an increased risk for anastomotic leaks [33, 43].
Other substances that have been used in the peritoneal cavity in an attempt to reduce adhesion formation include high-molecular-weight dextran, oxidized regenerated cellulose (Interceed), fibrin sealant, and hydrogel. The only substance that has shown a consistent reduction in adhesions in clinical trials has been Interceed [43].
Inflammatory adhesions
Episodes of intra-abdominal inflammation, including, but not limited to ovarian torsion, ventriculoperitoneal shunt infection, Crohn's disease, acquired immunodeficiency syndrome, and pelvic inflammatory disease, can lead to adhesion formation and subsequent intestinal obstruction in the absence of previous surgical procedures.
Postoperative paralytic ileus
Postoperative paralytic ileus (PPI) refers to obstipation and intolerance of oral intake due to non mechanical factors that disrupt the normal coordinated propulsive motor activity of the gastrointestinal tract following abdominal or non abdominal surgery.
The pathogenesis of PPI is multifactorial, and includes activation of inhibitory reflexes, inflammatory mediators and opioids (endogenous and exogenous).
In the majority of patients, PPI resolved within 5 to 7 days. Passage of flatus signifies the return of colonic function and usually indicates that the ileus has resolved [43].
NB: For patients with protracted ileus, mechanical obstruction must be excluded with contrast studies (barium enema or upper GI series).
Prevention
The duration of PPI is prolonged by use of narcotics in a dose-dependent manner. Opiates, excessive trauma to the bowel, intra-abdominal bleeding, and preoperative gastric obstruction also prolong the return of normal bowel function.
Сontinuous postoperative epidural analgesia including local anaesthetics has been most effective in the prevention of PPI. Choice of anaesthetic technique has no major impact on PPI.
NB: Thoracic epidural anesthesia with local anesthetics increased splanchnic blood flow, impeded afferent and efferent inhibitory reflexes, and when administered in the thoracic region, has demonstrated a significant reduction in the duration of PPI.
Lumbar epidural administration failed to have a similar effect on PPI [3, 43].
Minimally invasive surgery reduces PPI, in accordance with the sustained reduction in the inflammatory responses, while the effects of early institution of oral nutrition on PPI are minor [16, 43].
Treatment
Postoperative ileus is a significant problem with multiple causes, and thus treatment should be multimodal. The management may vary greatly depending on the nature of the disease and the surgical procedure. Most treatment is supportive. No single objective variable accurately predicts the resolution of ileus.
The first step in treating ileus is refraining from eating or drinking.
Patients should receive intravenous hydration. Management starts with correction of underlying medical conditions, electrolyte abnormalities, and acid base abnormalities.
Patients might be given enemas to stimulate a bowel movement.
Several pharmacological agents have been employed to resolve PPI (propranolol, dihydroergotamine, neostigmine, erythromycin, cisapride, metoclopramide, cholecystokinin, ceruletide and vasopressin), most with either limited effect or limited applicability because of adverse effects [16, 43].
For patients with vomiting and distention, use of a nasogastric tube provides symptomatic relief; however, no studies in the literature support the use of nasogastric tubes to facilitate resolution of ileus. Long intestinal tubes have no benefit over nasogastric tubes.
Physical activity can be one of the most helpful solutions in treating ileus.
Recent attempts to postoperatively stimulate the cephalic-vagal axis through sham-feedings (chewing of gum) have been associated with improved outcomes but require further investigation [43].
Other Causes of Intestinal Obstruction
Volvulus
¤ Midgut Volvulus
Acute midgut volvulus is a true surgical neonatal emergency. Midgut volvulus is present in up to 50% of patients operated on for malrotation [45].
Intestinal malrotation is a congenital anomaly of rotation of the midgut (embryologically, the gut undergoes a complex rotation outside the abdomen).
On this video you can see a nomal rotation of the midgut:
NB: The presentation of midgut volvulus commonly begins with the sudden onset of bilious vomiting in a previously healthy newborn [36, 45].
Bilious emesis is the cardinal feature of neonatal intestinal obstruction, and mandates urgent evaluation to exclude malrotation with volvulus. Although bilious vomiting may be due to non surgical disorders such as sepsis, intracranial hemorrhage, and electrolyte abnormalities, malrotation must be rapidly excluded from the differential diagnosis in order to prevent significant morbidity and mortality.
Occult or gross blood in the stool may be due to intestinal ischemia and mucosal injury.
Initially, the abdomen is nondistended, but persistent intestinal obstruction and vascular insufficiency leads to abdominal distention and tenderness with lethargy and other signs of shock.
Abdominal radiographs initially may not be diagnostic [36, 45].
A high index of suspicion is important because the complications of vascular compromise can advance rapidly. If the history and physical findings are highly suspicious for acute midgut volvulus, urgent operative intervention is indicated without confirmatory radiographic studies. This is justified due to the disastrous consequences related to delayed treatment of this potentially correctable process.
A midgut volvulus may have an atypical presentation, primarily in older patients. The volvulus may be intermittent or incomplete and chronic. Common clinical findings include chronic abdominal pain, intermittent vomiting, weight loss, failure to thrive, malabsorption, and diarrhea. Vomiting is either bilious or nonbilious. Chronic volvulus causes partial vascular obstruction, resulting in mucosal malabsorption, protein loss, ischemia, or hemorrhage. Physical findings may be completely unremarkable, but blood in the stool may be detected. The diagnosis is confirmed radiographically by an upper GI series [14, 45].
¤ Volvulus of Large bowel
Although not an uncommon etiology of large bowel obstruction in adults, colonic volvulus is a rare entity in children.
These children have an acute onset of low bowel obstruction. Abdominal radiographs demonstrate dilated loops proximal to the obstruction, and barium enema is diagnostic by demonstrating the characteristic "bird's beak" appearance in the involved bowel segment.
Treatment options are sigmoidoscopy or colonoscopy, laparoscopy, or primary laparotomy with distorsion as the goal. Resection plus end-to-end anastomosis is the treatment of choice for volvulus occurring in the transverse colon, splenic flexure, and sigmoid colon. In all cases, nonoperative reduction alone is not recommended because of the high risk of recurrence [43].
Herniations
Although hernias account for only 10% of small bowel obstructions, they are more likely to be associated with strangulation of the bowel [43]. Such hernias include inguinal, ventral, and internal hernias. Internal hernias, caused by internal bands or defects in the mesentery after bowel resection, are likely to cause a "closed-loop" bowel obstruction.
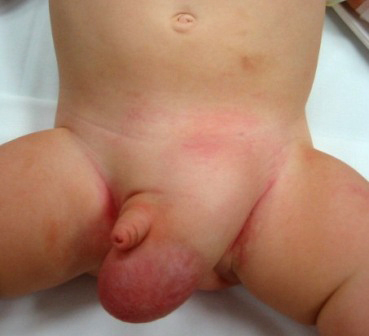
Incarcerated Inguinal Hernias
Inguinal hernia is a common type of ventral hernia that occurs when an intra-abdominal structure, such as bowel or omentum, protrudes through a defect in the abdominal wall. Most hernias that are present at birth or in childhood are indirect inguinal hernias. Other less common types of ventral hernias include umbilical, epigastric, and incisional hernias.
NB: When obliteration of the processus vaginalis fails to occur, inguinal hernia results.
All pediatric inguinal hernias require operative treatment to prevent the development of complications, such as inguinal hernia incarceration or strangulation.The incidence of incarcerated or strangulated hernias in paediatric patients is 10-20%. 50% of these occur in infants aged younger than 6 months [14, 43].
Irreducible abdominal hernias or incarcerated hernias may be painful, but their most relevant symptom is that they cannot return to the abdominal cavity when pushed in. They may be chronic, although painless, and can lead to strangulation (loss of blood supply) and/or obstruction (kinking of intestine). Strangulated hernias are always painful and pain is followed by tenderness. Nausea, vomiting, or fever may occur in these cases due to bowel obstruction. Also, the hernia bulge in this case may turn red, purple or dark and pink.
An irreducible hernia - also known as an incarcerated hernia - is a hernia that cannot be pushed back, manually, through the opening in the abdomen. An irreducible hernia is trapped outside the abdomen muscle wall.
A strangulated hernia is a surgical emergency in which the blood supply to the herniated tissue is compromised. Strangulation reduces venous return and leads to increased tissue edema, which further compromises circulation and stops the arterial supply.
Differential Diagnosis
- · Hydrocele of spermatic cord (spermatic cord cyst)
- · Testicular torsion
- · Inguinal lymphadenitis
A strangulated hernia is a medical emergency that requires immediate surgery. Age limits do not take into account. Manual reduction of inguinal hernia is unacceptable.
Patients presenting with an incarcerated hernia are first managed with nonsurgical attempts at reduction unless they demonstrate evidence of local or generalized sepsis suggestive of ischemia or perforation of incarcerated intestine [1, 3, 6, 14].
During hospitalization of the child with incarcerated hernia:
- · With the aim of preoperative preparation administered analgesics and 0.1% solution of atropine
- · Child is placed in a warm, dark, restful environmen
- · 30-40 ° Trendelenburg position
Wait around 30 minutes. Some hernias self-reduce because the force of gravity, and relaxation of the muscles surrounding the hernia from sedation and analgesia
If there is no reduction of hernia itself (within 1-2-hours) – this is indication to manual reduction (no more than 2 attempts)!
Gentle attempts at reduction are made.
Manual procedure is effective - elective surgery is indicated.
Manual procedure is not effective - emergency surgery is indicated.
Complication
Manual reduction can be complicated by worsened pain secondary to pressure and manipulation.
The bowel progresses to obstruction and strangulation despite apparent reduction.
If strangulation is not recognized, gangrenous bowel can be reduced, which leads to peritonitis and sepsis.
Surgery
The standard inguinal incision is made initially.
Surgical technique's features of incarcerated and strangulated inguinal hernia [4, 7, 8]:
- Dissection of the hernia sac and manual fixation of hernia's content than dissection infringes ring. The edematous sac is first freed from the cord structures and opened.
- Prevent of spontaneous reduction hernia content.
- The bowel is carefully inspected to assess viability prior to returning it into the abdominal cavity.
- Nonviable bowel is resected and primary anastomosis performed.