-
Aortic Stenosis
-
Pulmonary valve stenosis
-
D-Transposition
of the great arteries
-
L-Transposition
of the great arteries
Aortic Stenosis
The clinical spectrum of congenital aortic stenosis varies from normal function of a malformed bicuspid aortic valve to severe aortic stenosis in fetal life resulting in hypoplastic left heart syndrome. Congenital aortic stenosis occurs in different forms, classified with respect to the location of the obstruction relative to the aortic valve: Valvular, subvalvular, or supravalvular.
PREVALENCE
Left ventricular outflow tract (LVOT) obstruction, which includes stenosis at, below, or above the aortic valve, represents up to 10% of all CHDs. Valvular AS is the most frequent (71%), followed by subvalvular stenosis (23%) and supravalvular stenosis (6%). Aortic valve stenosis occurs more often in males (male/female ratio of 4:1).
-
Stenosis may be at the valvular, subvalvular, or supravalvular level.
-
Valvular AS may be caused by a bicuspid aortic valve, a unicuspid aortic valve, or stenosis of the tricuspid aortic valve. A bicuspid aortic valve with a fused commissure and an eccentric orifice accounts for the most common form of aortic valve stenosis (75%). Less common is the unicuspid valve with one lateral attachment. A valve that has three unseparated cusps with a stenotic central orifice is the least common form. Many bicuspid aortic valve are nonobstructive during childhood and become stenotic in adult life because of calcification of the valve.
-
Symptomatic neonates with so-called critical neonatal aortic valve stenosis have primitive, myxomatous valve tissue, with a pinhole opening. The aortic valve and ascending aorta are almost always hypoplastic. Hypoplasia of the mitral valve, LV cavity, or LV outflow tract and a VSD are also frequently found, often requiring one ventricular repair (Norwood and Fontan operations).
-
Supravalvular AS is an annular constriction at the upper margin of the sinus of Valsalva. Occasionally, the ascending aorta is diffusely hypoplastic. This is often associated with Williams' syndrome (which includes mental retardation, characteristic facies, and multiple PA stenosis).
-
Subvalvular (subaortic) stenosis may be in the form of a simple diaphragm (discrete) or a long, tunnel-like fibromuscular narrowing (tunnel stenosis) of the left ventricular outflow tract.
-
Discrete membranous subaortic stenosis accounts for about 10% of all AS cases, and it occurs more often than tunnel stenosis. It is believed to develop as the result of turbulence in an abnormally shaped LVOT, which causes endocardial injury and subsequent proliferation and fibrosis.
-
Two thirds of the patients have associated cardiac lesions, such as VSD, PDA, or COA.
-
In one third of the patients, the stenosis is isolated; familial subaortic membrane has been reported.
-
In some patients, there is history of surgical intervention, such as membranous VSD closure or PA banding (9 months to 8 years before the development of the membrane).
-
-
Tunnel-like subaortic stenosis is often associated with hypoplasia of the ascending aorta and aortic valve ring, as well as thickened aortic valve leaflets. It is usually associated with other LV anomalies, including Shone's complex (comprising supramitral ring, parachute mitral valve, subaortic stenosis, and COA).
-
Another type of subvalvular stenosis is idiopathic hypertrophic subaortic stenosis (see Fig. 1E ), a primary disorder of the heart muscle.
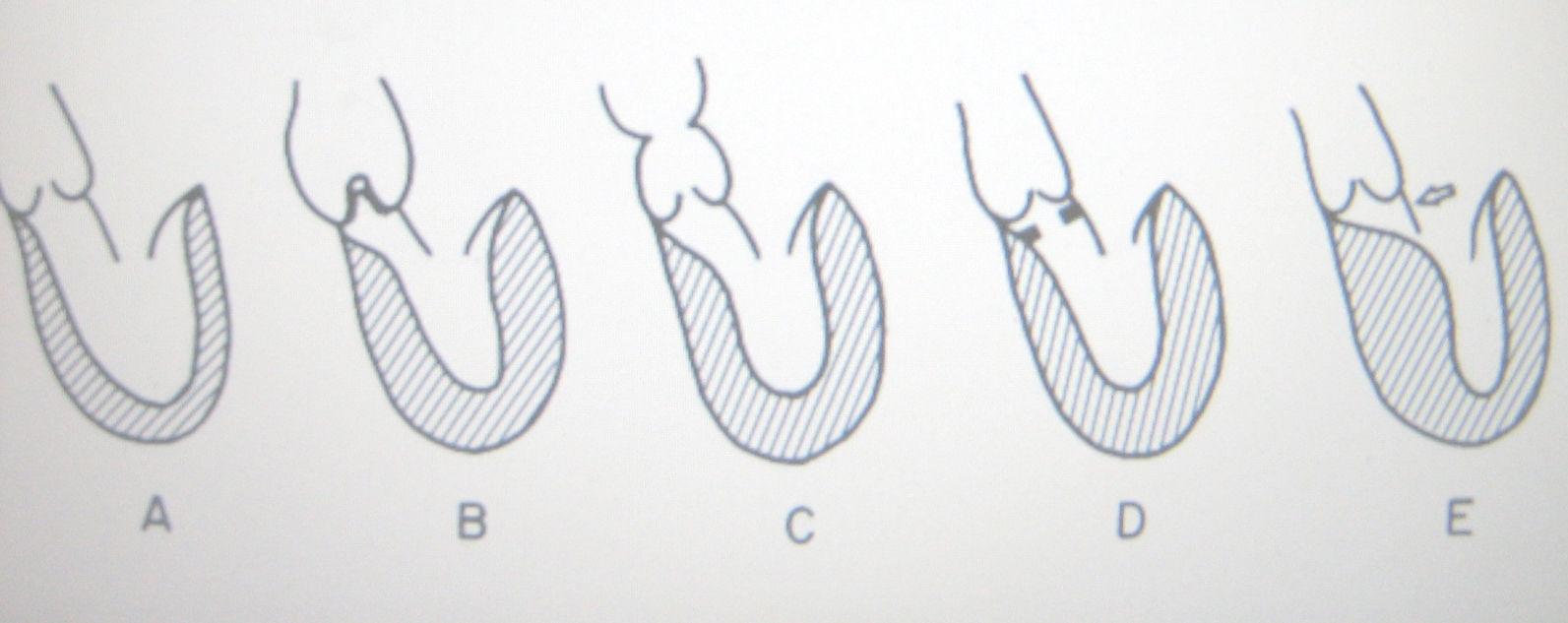
Figure 1 Anatomic types of aortic stenosis. A, Normal. B, Valvular stenosis. C, Supravalvular stenosis. D, Discrete subaortic stenosis. E, Idiopathic hypertrophic subaortic stenosis
Pathophysiology. Left ventricular outflow obstruction causes a rise in the systolic pressure in the left ventricle that is proportional to the degree of obstruction. The resulting left ventricular hypertrophy and the high intracavitary pressure may lead to inadequate coronary filling and result in a mismatch between myocardial supply and demand. Left ventricular end-diastolic pressure may become elevated if left ventricular function is impaired or if hypertrophy is severe enough to reduce compliance.
CLINICAL MANIFESTATIONS
History
-
Neonates with critical or severe stenosis of the aortic valve may develop signs of hypoperfusion or respiratory distress related to pulmonary edema within days to weeks after birth.
-
Most children with mild to moderate AS are asymptomatic. Occasionally, exercise intolerance may be present.
-
Exertional chest pain, easy fatigability, or syncope may occur in a child with a severe degree of obstruction.
Physical Examination
-
Infants and children with AS are acyanotic and are normally developed.
Electrocardiography.
In mild cases the ECG is normal. ECG may show right ventricular hypertrophy, left ventricular hypertrophy, and/or evidence of myocardial ischemia. Left ventricular ischemia, shown by ST-segment depression and T-wave inversion, is indicative of severe stenosis, but absence of left ventricular ischemia does not exclude severe stenosis.
X-ray Studies
|
1. |
The heart size is usually normal in children, but a dilated ascending aorta or a prominent aortic knob may be seen occasionally in valvular AS, resulting from poststenotic dilatation. |
|
2. |
Significant cardiomegaly does not develop unless CHF occurs later in life or if AR becomes substantial. |
|
3. |
Newborns with critical AS show generalized cardiomegaly with pulmonary venous congestion. |
Echocardiography
Echocardiogram is diagnostic. The site of the lesion, the degree of obstruction, and the size and function of the left ventricle all can be determined. Pressure gradient across the aortic valve can be calculated by continuous-wave Doppler ultrasonography but may be misleadingly low and not indicative of the severity of the stenosis in the presence of a very low cardiac output.
Cardiac catheterization.
Cardiac catheterization is not necessary for diagnosis. The pressure gradient can be measured but with the low cardiac output the degree of obstruction may be underestimated. The major benefit of angiography is the definition of associated arch anomalies.
Therapy
(a) Medical management. Bacterial endocarditis prophylaxis is indicated.
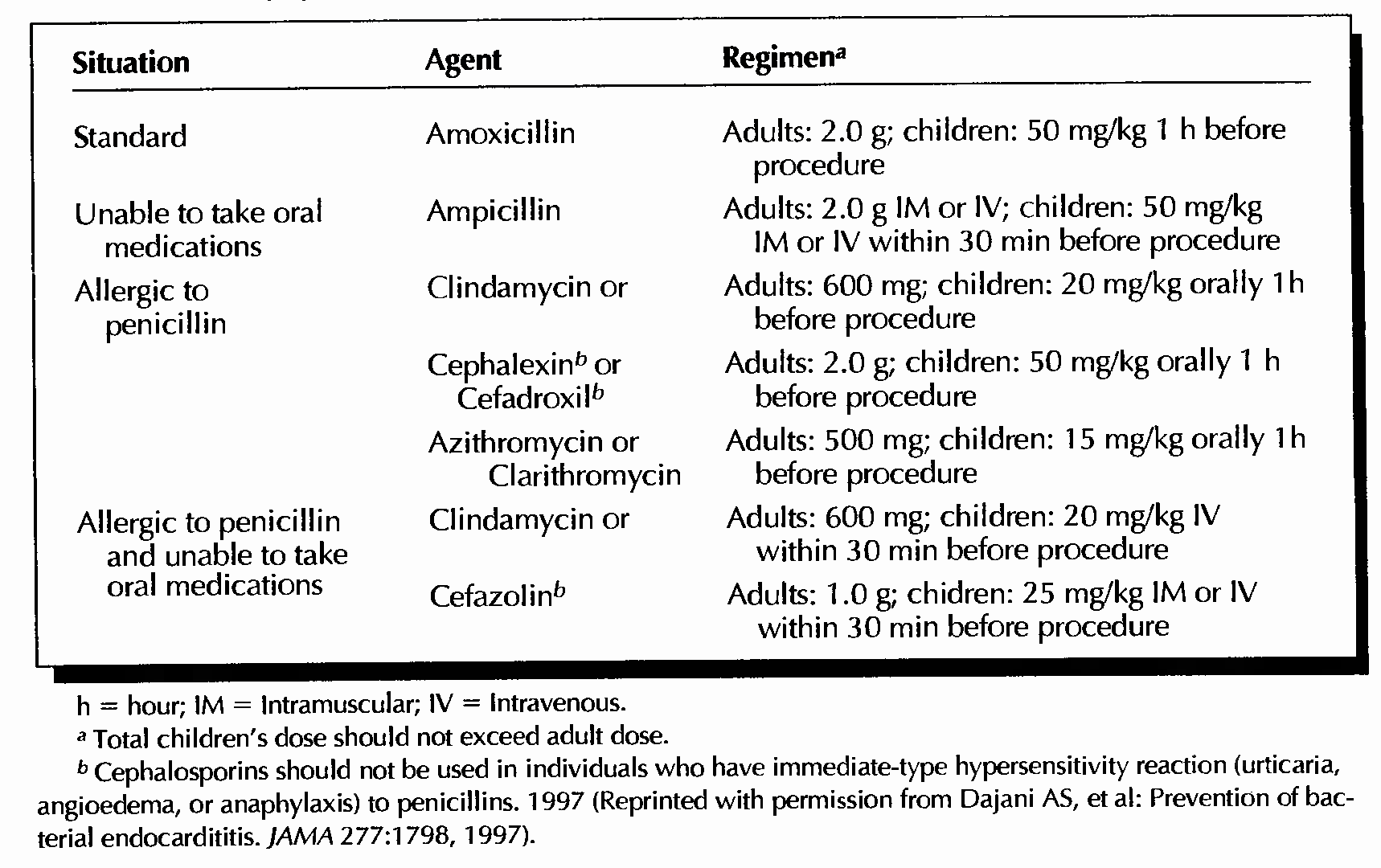
Avoidance of competitive sports in all but the mildest cases is usually recommended. Hemodynamic stabilization is achieved by reopening the ductus arteriosus with PGE1 infusion, using inotropic agents and diuretics, and correcting acid-base imbalances. Balloon angioplasty of the aortic valve can be lifesaving. Balloon angioplasty of the aortic valve can decrease the severity of stenosis and significantly diminish the transvalve gradient.
(b) Surgical management. In selected patients with aortic valve stenosis, open valvotomy or aortic valve replacement must be performed. Aortic valvotomy under direct visualization is associated with a high mortality rate.
Pulmonary valve stenosis
Description. Pulmonary valve stenosis accounts for 5% to 8% of congenital heart defects. The pulmonary commissures are fused, the valve is domed, and there is post-stenotic dilatation of the main pulmonary artery. The pulmonary valve is occasionally bicuspid and is dysplastic in 10% of patients.
Pathophysiology. To maintain cardiac output, right ventricular pressure rises. In severe stenosis, right ventricular end-diastolic pressure may also increase. A consequent increase in right atrial pressure may open the foramen ovale and cause a right-to-left shunt.
Clinical features. Most patients are asymptomatic. Severe to critical pulmonary stenosis may cause exertional dyspnea, fatigability, and exertional chest pain. Congestive heart failure is unusual except in infants who have critical stenosis.
Diagnosis
a. Physical examination
- ) An ejection click, the loudness of which varies with respiration, and a harsh systolic ejection murmur are present at the upper left sternal border.
- ) In moderately severe stenosis, a thrill and right ventricular heave are palpable; the pulmonary component of S2 is diminished; the ejection click merges with S,; and the murmur becomes longer and louder.
- ) If the stenosis is critical, cyanosis may become evident and an S4 gallop may be heard.
b. Laboratory evaluation
- ) Chest radiograph. Heart size and pulmonary vascularity are usually normal, but the pulmonary artery segment is prominent because of poststenotic dilatation. In critical stenosis, cardiomegaly and diminished pulmonary blood flow may be seen.
- ) ECG. The degree of right-axis deviation and right ventricular hypertrophy correlates well with right ventricular pressure and, therefore, with severity of the stenosis. An R wave of 20 mm or greater in lead V, or a positive T wave in the right precordial leads is indicative of systemic right ventricular pressure.
- ) Echocardiogram. Right ventricular hypertrophy, or dilatation, doming of the pulmonary valve, and poststenotic dilatation of the pulmonary artery can all be seen. The integrity of the interatrial septum can be assessed. Doppler ultrasonography can estimate the transvalve gradient.
- ) Cardiac catheterization. Right ventricular function and the transvalve gradient can be accurately assessed. Severity of stenosis is defined by right ventricular pressure: In mild stenosis, right ventricular pressure is less than 50% of systemic pressure; in moderate stenosis, it is 50% to 80% of systemic pressure; and in severe stenosis, it is greater than 80% of systemic pressure.
Therapy
a. Medical management. Bacterial endocarditis prophylaxis is necessary. Percutaneous balloon angioplasty of the pulmonary valve may be performed at the time of cardiac catheterization and is highly effective.
b. Surgical management. Surgical resection of the pulmonary valve is reserved for those patients in whom balloon angioplasty has failed, including patients who have dysplastic valves.
D-Transposition of the great arteries
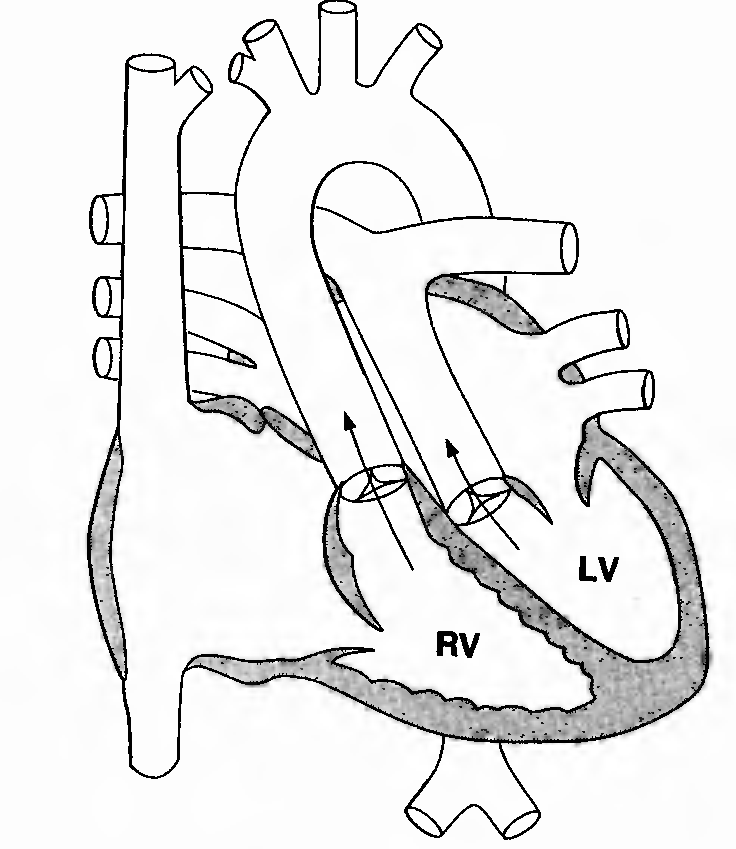 FIGURE 1. Anatomy of D-transposition of the great arteries. Arrows
indicate the usual direction of flow. RV = right ventricle; LV = left
ventricle.
FIGURE 1. Anatomy of D-transposition of the great arteries. Arrows
indicate the usual direction of flow. RV = right ventricle; LV = left
ventricle.
Description. This lesion, also known as simple transposition, accounts for 5% of congenital heart defects and is more common in boys than in girls. The aorta arises from the right ventricle anteriorly and to the right of the pulmonary artery, which arises posteriorly from the left ventricle. Associated abnormalities may include VSD, PDA, pulmonary stenosis, or a combination of these.
Pathophysiology. Systemic venous (unoxygenated) blood is recirculated through the body, and pulmonary venous (oxygenated) blood is recirculated through the lungs. A lesion that allows mixing of the systemic and pulmonary circulations (e.g., ASD, VSD, PDA) is necessary for survival.
Clinical features. Cyanosis is present from birth, the degree varying with the associated mixing lesion.
Diagnosis
a. Physical examination. Intense cyanosis is noted in the absence of mixing lesions. In addition, a right ventricular heave and a single loud S2 are usually found, and a soft flow murmur may be heard.
b. Laboratory evaluation
- ) Chest radiograph. Pulmonary vascularity is increased or may be normal. Slight cardiomegaly and a narrow base produced by the anterior- posterior arrangement of the great arteries give the heart the shape of an egg on its side.
- ) Arterial blood gas analysis shows severe hypoxemia (PO2 is often in the low 20s); increasing the ambient FIO2 to 100% does not significantly alter the arterial PO2.
- ) ECG findings are normal in the newborn.
- ) Echocardiogram shows the anterior-posterior arrangement of the great arteries and the chamber from which they originate, the normal anatomy of the ventricles, and the presence of associated abnormalities. Color flow mapping shows the direction of flow through associated abnormalities.
- ) Cardiac catheterization. Right ventricular pressure is systemic. Left ventricular pressure may be systemic in the newborn but decreases with a decline in pulmonary vascular resistance. Angiography confirms the anatomy and indicates the direction of blood flow. Balloon atrial septostomy (Rashkind procedure) allows creation of an ASD, through which the mixing of oxygenated and deoxygenated blood can occur.
Therapy
a. Medical management. Creation of an ASD by balloon atrial septostomy may be life- saving. Correction of acidosis, hypoglycemia, and hypocalcemia in the neonatal period improves myocardial function.
b. Surgical management. An arterial switch procedure with coronary artery re-implantation is best performed in the first 2 weeks of life and involves moving the arteries, but not the valves, into their "normal" position. Two atrial switch procedures, the Senning and Mustard, have been used in the past.
L-Transposition of the great arteries
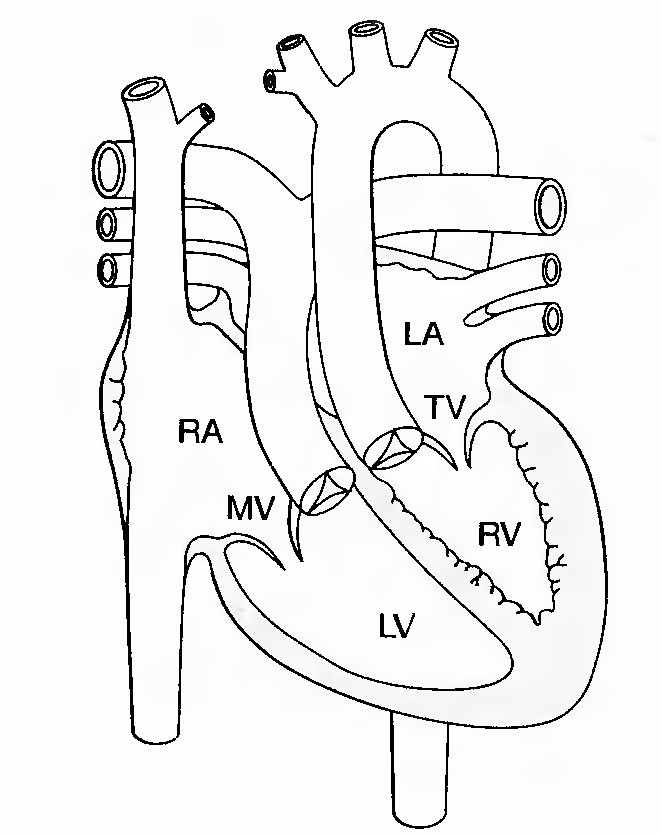 FIGURE
2. Anatomy of L-transposition of the great arteries. RA = right
atrium; RV = right ventricle; LA = left atrium; LV = left ventricle;
MV = mitral valve; TV = tricuspid valve.
FIGURE
2. Anatomy of L-transposition of the great arteries. RA = right
atrium; RV = right ventricle; LA = left atrium; LV = left ventricle;
MV = mitral valve; TV = tricuspid valve.
Description. L-Transposition of the great arteries accounts for less than 1% of all congenital heart disease. The great arteries are transposed, with the aortic valve anterior to and to the left of the pulmonary valve. In 98% of patients, the bulboventricular loop has developed to the left, which leads to inversion of the ventricles and atrioventricular discordance. The anatomic right ventricle is on the left, and the anatomic left ventricle is on the right (SLL). The AV valves are also inverted; the mitral valve leads into the anatomic left ventricle, and the tricuspid valve leads into the anatomic right ventricle. Atrial position is unaffected. Associated abnormalities are common and may include AV conduction block, VSD, pulmonary stenosis, and left-sided AV (i.e., tricuspid) valve in sufficiency.
Pathophysiology. The combination of L-transposition of the great arteries and inversion of the ventricles leads to a physiologically corrected circulation. (This lesion has also been called "corrected" transposition.) Systemic venous return flows via the mitral valve to the right-sided left ventricle and is ejected into a posterior pulmonary trunk; pulmonary venous return flows via the tricuspid valve to the left-sided right ventricle and from there to the anterior aorta. This corrected circulation may be altered by associated defects.
Clinical features. Symptoms reflect the associated defects.
a. A large VSD produces symptoms of a large left-to-right shunt, including congestive heart failure in infants.
b. Severe pulmonary valve or subvalve stenosis associated with a VSD produces symptoms of a right-to-left shunt (i.e., cyanosis, dyspnea), similar to those seen in tetralogy of Fallot.
c. Left-sided (tricuspid) valve regurgitation may produce tachypnea, dyspnea, cough, and other signs of pulmonary venous congestion.
d. Complete heart block may be associated with syncope and clinical evidence of low cardiac output.
Diagnosis
a. Physical examination
- ) In the absence of associated defects, the only physical finding that gives a hint of the underlying lesion is a loud single S2.
- ) If a VSD is present, the findings are similar to those noted previously.
- ) When severe pulmonary stenosis is associated with a large VSD, the findings are those of tetralogy of Fallot.
- ) Left atrioventricular valve (tricuspid valve) insufficiency is indistinguishable from severe mitral valve regurgitation.
b. Laboratory evaluation
1) Chest radiograph
a) Because the left heart border is formed by the ascending aorta, it is straight or slightly convex.
b) Cardiomegaly, pulmonary hyperflow, and left atrial enlargement are seen in the presence of a large VSD.
c) If severe pulmonary stenosis coexists with a VSD, the heart size is normal, and pulmonary vascularity is diminished.
d) Severe left-sided AV valve regurgitation results in an enlarged left atrium and evidence of pulmonary venous congestion.
- ) ECG. Inversion of the septum causes reversal of initial depolarization, which is indicated by deep Q waves in leads II, III, and aVf as well as in the right precordial leads. Varying degrees of AV block may be seen.
- ) Echocardiogram demonstrates ventricular structure, great vessel location, and associated defects. Color flow mapping indicates AV valve regurgitation and direction of flow through a VSD, and Doppler ultrasonography estimates the severity of pulmonary stenosis.
- ) Cardiac catheterization. In the absence of associated abnormalities, right-sided left ventricular pressure is low, and left-sided right ventricular pressure is systemic. Oxygen saturations and pressures are modified by associated defects, which are accurately delineated by angiography.
Therapy
a. Medical management is determined by the presence of associated cardiac defects. Measures include treatment of congestive heart failure and conduction disturbances as well as implementation of bacterial endocarditis prophylaxis.
b. Surgical management
1) Palliative systemic artery-pulmonary artery shunts relieve cyanosis produced by diminished pulmonary blood flow associated with VSD and severe pulmonary stenosis.
2) Pacemaker implantation in symptomatic bradycardia, surgical repair of VSD and pulmonary stenosis, and annuloplasty or replacement of a severely regurgitant left-sided AV valve are also surgically feasible.
