- Emergencies in Pediatric Urology
Emergencies in Pediatric Urology
Urolithiasis and Renal colic
Urolithiasis (from Greek oûron, "urine" and lithos, "stone") is the condition where urinary calculi are formed in the urinary tract.
The term kidney stone (or "renal calculus") is sometimes used to refer to urolithiasis in any part of the urinary tract; however it is more properly reserved for stones that are actually in the collecting duct of the kidney itself.
The term nephrolithiasis can be used to describe the condition of having kidney stones, and ureterolithiasis can be used to describe the condition of having stones in the ureter.
- Obstruction of the ureter by the kidney stones causes a renal colic attack which is why intense pain is felt in groin and back.
Stone disease describes a condition in which chemicals in the urine crystallize into "stones" in the urinary tract. These stones can cause pain by creating an obstruction of the drainage of the urine. There are many different kinds of urinary tract stones, with calcium, oxalate, uric acid, and phosphate being the most common components (Table 8.1).
Stones may cause problems anywhere in the urinary tract.
Table 8.1 Commonly occurring urinary tract stones and describes their salient features [39]
|
Composition |
Frequency |
Radiographic Appearance |
Associated Etiologic Factors |
|
Calcium oxalate monohydrate and dihydrate (calcium oxalate dihydrate)
|
40-60% |
Radiopaque |
Underlying metabolic disorder (eg, idiopathic hypercalcuria or hyperoxaluria) |
|
Hydroxy apatite (calcium phosphate)
|
20-60% |
Radiopaque
|
Usually no metabolic abnormality
|
|
Struvite
|
5-25 % |
Radiopaque |
Renal infection
|
|
Uric acid
|
5-10% |
Radiolucent |
Idiopathic hyperuricemia or hyperuri-cosuria |
|
Cystine
|
5-6% |
Mildly opaque |
Renal tubular defect |
|
Brushite
|
2-4% |
Radiopaque
|
--- |
Pathophysiology
Stones are not common in children, but the incidence of stones in children does seem to be increasing [30].
Although there are a number of contributing factors, the central concept in urolithiasis is urinary supersaturation. Supersaturation occurs when crystal precipitation occurs in the urine in the form of nucleation, the basis of urinary stones. Crystallization and aggregation must also occur for stones to form. These processes are influenced by the presence of inhibitors and promoters. Citrate, magnesium, pyrophosphate, glycosaminoglycans, nephrocalcin, and Tamm-Horsfall proteins are inhibitors of crystallization and aggregation.
Bacterial infections and anatomic abnormalities such as obstruction or stasis may encourage crystal aggregation and retention, thus increasing the risk of clinically significant urolithiasis.
u Basically stones form because there is too much of the ingredients of the stone and not enough water in the urine. This can occur either because there is an abnormally high amount of stone-forming material in the urine, or the urine is too concentrated because of dehydration.
Renal, urologic, endocrine, and metabolic disorders may lead to the development of crystallized material in the urinary system.
Multiple studies of urolithiasis in children have shown metabolic abnormalities in up to 92% of patients, with hypercalciuria and hypocitraturia the most common.
The prevalence of infection-related stones is especially high in children younger than 6 years. Approximately 12% of children with urolithiasis have no identifiable risk factor (idiopathic).
NB: Urinary stone formation is the result of a complex process involving metabolic, anatomical factors and presence of infection [30].
Classification
Urinary stone disease can, therefore, be classified as metabolic, anatomic, infectious, or idiopathic, on the basis of underlying factors.
Stones can also be classified on the basis of their location in the upper urinary tract (kidneys and ureters) or lower urinary tract (bladder and urethra) and as symptomatic or asymptomatic. These classifications may influence the decision-making process with regard to treatment options.
Stones are most often classified into groups based on their chemical components (See Table 8.1). Materials that produce stones in the urinary tract of children include (with the approximate frequency):
1. calcium with phosphate or oxalate – 20-60%
2. magnesium ammonium phosphate (struvite or infection stons) – 5-25%
3. uric acid – 5-10%
4. cysteine – 5-6%
5. combinations of the preceding items (mixed) – 2%
6. purine derivatives
7. drugs or their metabolites (eg, phenytoin, triamterene).
Epidemiology
Stone formation in urinary tract affects approximatly 5-10% of the population. In children, stone disease is less common than it is in adults. Children account for just 1-3% of all stone patients [16]. The incidence of stone disease in children is increasing [30].
The incidence of urolithiasis shows marked geographical variation. It is rare in children in industrialized countries. Bladder stones are more common in boys in less developed nations, particularly in the Middle East and Asia [16, 43].
Peak presentation for adults is middle age. Children can present with stones at any age (eg, premature newborn to teenager).
In children, calcium stones are most common (adults also are most often afflicted with calcium oxalate or calcium phosphate stones).
In some cases, the primary cause of stone formation cannot be identified.
Stones are more frequent in men than in women (4:1), although the boy-to-girl ratio (3:2) is closer to equal.
Clinical presentation
In adults, upper tract calculi present in a characteristic fashion in the form of renal colic. This severe, intermittent, refractory pain, often accompanied by nausea and vomiting, is a less common presentation in children.
The most common symptoms of kidney stones include:
· Pain in the belly or back
· Blood in the urine (hematuria)
· Nausea or vomiting
· Needing to rush to the bathroom to urinate
NB: Some children, particularly young children, do not have any symptoms, and the kidney stone is found when an imaging test (like an X-ray) is done for another reason.
Pain is noted in 50% of children with urolithiasis. The pain can localize to the upper abdomen, flank, or pelvis and may radiate to the umbilicus or groin. Associated nausea and vomiting are not uncommon.
Hematuria may be noted in 30% to 90% of children with stones, but gross hematuria is relatively uncommon [43].
Younger children in particular may also present with nonspecific abdominal pain accompanied by microscopic hematuria or, less frequently, with outlet obstruction.
Some kidney stone symptoms are similar to those of a bladder infection.
Diagnosis
History and physical examination are key components to rule out other causes of abdominal and back pain. The history should include a dietary and urologic history.
Laboratory Studies*
§ Urinalysis – microscopic haematuria may be the sole indicator and is more common in children [30].
§ Complete blood count (CBC)
§ Blood chemistry tests
* About laboratory studies see below in section “Metabolic evaluation”.
Imaging
¤ Generally, ultrasonography should be used as a first study. Renal ultrasonography is very effective for identifying stones in the kidney [3, 24, 30, 43].
¤ Approximately 90% of urinary tract stones are radioopaque and can be seen on plain radiographs. Many radiopaque stones can be identified with a simple abdominal plain X-ray [3, 8].
¤ If no stone is found but symptoms persist, spiral CT scanning is indicated. The most sensitive test for identifying stones in the urinary system is non-contrast helical CT scanning. It is safe and rapid, with 97% sensitivity and 96% specificity [24, 30].
¤ Intravenous pyelography (IVP) is rarely used in children, but may be needed to delineate the caliceal anatomy prior to percutaneous or open surgery [30].
Metabolic evaluation
Due to the high incidence of predisposing factors for urolithiasis in children and high stone recurrence rates, every child with urinary stone should be given a complete metabolic evaluation [30].
Metabolic evaluation includes:
· Family and patient history of metabolic problems. One clue to the presence of urolithiasis is a positive family history for kidney stones, which may be present in up to 37% of children with stone disease [43].
· Analysis of stone composition (See Addition F). Following stone analysis, metabolic evaluation can be modified according to the specific stone type.
· Electrolytes, blood urea nitrogen, creatinine, calcium, phosphorus, alkaline phosphatase, uric acid, total protein, carbonate, albumin, and parathyroid hormone (if there is hypercalcaemia).
· Spot urinalysis and culture, including ratio of calcium to creatinine.
· Urine tests, including a 24-hour urine collection for calcium, phosphorus, magnesium, oxalate, uric acid citrate, cystine, protein, and creatinine clearance.
Treatment
Four main factors affect initial treatment decisions: the clinical scenario, stone composition, stone size, and stone location (Table 8.2-8.4).
During the acute phase (acute renal colic) when the stone is being passed, management is directed toward pain control and facilitating passage or removal of the stone(s).
Oral (or IV) fluids and analgesic should be encouraged to facilitate the passage of stones and to reduce any risk of permanent damage to renal function. Nonsteroidal anti-inflammatory drugs (e.g., Ibuprofen) and opioids can be cautiously used to control acute pain (as analgesic).
The use of tamsulosin has become a popular yet off-label use of a medication to assist in the passage of ureteral stones in adults (expulsive therapy). But the use of tamsulosin in the conservative treatment of distal ureteral stones in the pediatric population should be implemented with caution [43].
NB: Many stones in the renal tract will pass spontaneously but large ones may require removal.
Stone size can be used to predict whether the stone will pass without intervention. Therefore supportive care in the form of vigorous hydration (oral or intravenous, as needed) and analgesic therapy is a reasonable first step in a child with a small stone in the absence of fever or complete ureteral obstruction [30, 43].
Studies demonstrate that partial obstruction is well tolerated in the short term (several days); thus this treatment may be continued for 3 to 4 weeks on an patient basis to allow spontaneous passage of the stone [43]. During this time, the location of the stone is usually monitored with ultrasound.
If the child passes a stone or stone fragment, save it in a clean container. A lab can analyze the stone to determine the type, which can guide treatment.
After relief of the obstruction and subsequent treatment of infection, definitive therapy directed at stone clearance can be undertaken.
Table 8.2
Factors Affecting the Treatment of Stones [43]
|
Factor |
Treatment Consideration |
|
Clinical scenario |
|
|
Bilateral obstruction |
For all scenarios, urgent relief of obstruction via stent or nephrostomy |
|
Obstruction of a solitary kidney |
|
|
Fever/UTI with potential obstruction |
|
|
Intractable pain |
|
|
Stone composition |
|
|
Uric acid |
Consider chemodissolution |
|
Struvite |
Continue antibiotic therapy throughout treatment |
|
Cystine |
Responds poorly to ESWL |
|
Calcium oxalate |
Radiopaque; may respond well to ESWL |
|
Stone size |
|
|
<4 mm |
Approximately 90% chance of spontaneous passage in adults |
|
4-6 mm |
Approximately 50% chance of spontaneous passage in adults |
|
>6 mm |
Approximately 10%-20% chance of spontaneous passage in adults |
|
Stone location |
|
|
Renal |
ESWL or PCNL |
|
Proximal ureteral |
Ureteroscopic extraction (antegrade) or ESWL |
|
Distal ureteral |
Ureteroscopic extraction (retrograde) or ESWL |
Table 8.3
Recommendations for interventional management
in pediatric stones [30]
|
Stone size and localisation* |
Primary treatment option |
LE GR |
Secondary treatment options |
|
Staghorn stones |
PCNL |
2B |
Open/ESWL |
|
Pelvis < 10 mm |
ESWL |
1A |
RIRS/PCNL |
|
Pelvis 10-20 mm |
ESWL |
2B |
PCNL/Open |
|
Pelvis > 20 mm |
PCNL |
2B |
ESWL/Open |
|
Lower pole calyx < 10 mm |
ESWL |
2B |
RIRS/PCNL |
|
Lower pole calyx > 10 mm |
PCNL |
2B |
ESWL |
|
Upper ureteric stones |
ESWL |
2B |
PCNL/URS/ Open |
|
Lower ureteric stones |
URS |
1A |
ESWL/Open |
|
Bladder stones |
Endoscopic |
2B |
|
* - cysteine and uric acid stones excluded
PCNL - percutaneous nephrolithotomy; ESWL - extracorporeal shock-wave lithotripsy; RIRS - retrograde intrarenal surgery; URS – ureteroscopy; LE - level of evidence, GR - grade of recommendation.
NB: Staghorn calculi (also sometimes called coral calculi) obtain their characteristic shape, thus resembling the horns of a stag. Staghorn calculi are branched stones that occupy a large portion of the collecting system. Typically, they fill the renal pelvis and branch into several or all of the calices.
Uric acid stones may be dissolved and prevented by alkalinization of the urine (pH > 6.5) using sodium bicarbonate or potassium citrate. If symptoms are present, a ureteral stent or nephrostomy tube may be used for temporary relief during dissolution therapy [43].
Cystine stones are typically difficult to treat and frequently recur. Small renal cystine stones may be treated by ESWL, whereas larger stones will likely require PCNL or ureteroscopic extraction.
Struvite stones are usually associated with infection, and antibiotics should be continued throughout treatment. Because struvite stones are generally large or staghorn in shape, percutaneous nephrostolithotomy (PCNL) is often the first-line treatment for these stones.
Nonobstructing stones can be treated electively.
Treatment should be supported with medical treatment for the underlying metabolic abnormality if detected.
Table 8.4
Comments for interventional management
in pediatric stones [30]
|
Stone size and localisation* |
Comments |
|
Staghorn stones |
Multiple sessions and accesses with PCNL may be needed. Combination with ESWL may be useful. |
|
Pelvis < 10 mm |
|
|
Pelvis 10-20 mm |
Multiple sessions with ESWL may be needed. PCNL has similar recommendation grade. |
|
Pelvis > 20 mm |
Multiple sessions with ESWL may be needed. |
|
Lower pole calyx < 10 mm |
Anatomical variations are important for complete clearance after ESWL. |
|
Lower pole calyx > 10 mm |
Anatomical variations are important for complete clearance after ESWL. |
|
Upper ureteric stones |
|
|
Lower ureteric stones |
Additional intervention need is high with ESWL. |
|
Bladder stones |
Open is easier and with less operative time with large stones. |
Open surgery for stone disease in children is an exceedingly rare requirement. Surgical treatment is based on minimally invasive modalities. The use of appropriate-size instruments will decrease the number of complications in surgical treatment [30].
Recurrence and Kidney Stone Prevention
After the acute episode (acute renal colic), management is directed toward prevention of stone recurrence by reducing risk factors associated with stone formation.
Children who develop a kidney stone have a significant chance of developing stones in the future - 30-65% [43]. Therefore a thorough metabolic and anatomical evaluation is strongly encouraged in children after their first presentation with urolithiasis [30].
The cornerstones for preventing stone recurrence as the child enters adulthood are the ability to render the patient stone free, elucidate and treat metabolic abnormalities, control urinary infection, and correct anatomic anomalies [3, 8, 43].
The number of steps can decrease the chances of developing another stone:
u Drink more fluids – drinking more fluids can help to decrease the risk of forming all types of kidney stones. The goal is to increase the amount of urine that flows through the kidneys and ureters and to lower the concentration of substances that promote stone formation.
u The prevention advised will depend upon the chemical components of stones:
Calcium – children with increased levels of calcium in the urine should drink more fluids and make some changes in their diet:
· Eat a low-sodium diet
· Get the right amount of calcium from foods and drinks. Consuming too much calcium in foods and drinks is not recommended. However, the child should not stop eating foods and drinks with calcium because calcium is important in building strong bones.
The "right" amount of calcium depends on the child's age:
v 500 mg/day for children 1 to 3 years
v 800 mg/day for children 4 to 8 years
v 1300 mg/day for children 9 years and older
· Avoid calcium and vitamin D supplements
· Eat potassium-rich foods (fresh fruits and vegetables)
· If urine calcium levels are still high after 3 to 6 months of these changes, a medicine might be recommended.
Oxalate – children who have high levels of oxalate in the urine should:
· Drink more fluids
· Avoid vitamin C supplements
· Avoid foods that contain large amounts of oxalate, including beet and turnip greens, rhubarb, strawberries, star fruit, sweet potatoes, wheat bran, tea, cocoa, pepper, chocolate, parsley, beets, spinach, dill, nuts, and citrus juices
Urate – children with increased levels of urate in the urine should drink more fluids. Some children will be given a treatment to increase the pH of the urine (potassium citrate or potassium carbonate).
Cystine – children with high levels of cystine in the urine should drink more fluids. Some children will be given a medicine that reduces the acidity (i.e., increases the pH) of the urine (potassium citrate or potassium carbonate).
Low citrate – children who have a low level of citrate in the urine are usually given a treatment to increase citrate levels (potassium citrate or potassium bicarbonate).
Struvite – struvite stones usually develop because of a urinary tract infection. Preventing future urinary tract infections can help to prevent struvite stones.
Complementary and alternative therapies — there are no data about the safety or benefit of complementary and alternate therapies for kidney stones in children (including herbs, homeopathy, acupuncture, and others). Therefore the associations of pediatric urologists do not recommend these therapies because they are unproven.
Acute disease of scrotum (Acute scrotum)
The acute scrotum presents as acute painful swelling of scrotum or its contents.
The "acute scrotum" may be viewed as the urologist's equivalent to the general surgeon's "acute abdomen." Both conditions are guided by similar management principles:
- The patient history and physical examination are key to the diagnosis and often guide decision making regarding whether or not surgical intervention is appropriate.
- Imaging studies should complement, but not replace the clinical judgment.
- When making a decision for conservative, non-surgical care, the provider must balance the potential morbidity of surgical exploration against the potential cost of missing a surgical diagnosis.
- A small but real, negative exploration rate is acceptable to minimize the risk of missing a critical surgical diagnosis.
Diagnosis and Differential diagnosis
Acute scrotum is a pediatric urology emergency case, most commonly caused by torsion of the testis, torsion of the appendix testis and epididymitis/epididymoorchitis. Other causes of acute scrotal pain are idiopathic scrotal oedema, mumps orchitis, varicocele, scrotal haematoma, incarcerated hernia, appendicitis or systemic disease (Henoch-Schônlein purpura).
While the differential diagnosis is broad, an accurate history and physical examination can frequently precisely define the condition. Often, carefully chosen imaging studies can compliment clinical judgment and expedite therapeutic decisions.
Any acute scrotal pain requires immediate surgical assessment for torsion of the testis or strangulated inguinal hernia, which are surgical emergencies. In practice it is often difficult to be certain of the diagnosis clinically i.e. sometimes the diagnosis may only be made by surgical exploration.
The child's age suggests the cause of the acutely painful scrotum because torsion of the appendix testes/epididymis is more common in prepubertal boys and spermatic cord torsion (torsion of the testis) occurs more frequently in adolescents and newborns.
A list of potential medical conditions that can present as acute pain or swelling of the scrotum are found in Table 8.5
Table 8.5 Causes of Acute Scrotal Pain and Swelling (American Urological Association, 2013)
|
Ischemia:
|
|
|
Trauma:
|
|
|
Infectious conditions:
|
|
|
Inflammatory conditions:
|
|
|
Hernia:
|
Incarcerated, strangulated inguinal hernia, with or without associated testicular ischemia |
|
Acute on chronic events:
|
|
Table 8.6 Features on history and examination
|
Diagnosis |
Suggestive features on history |
Suggestive features on examination |
|
Torsion of the testis |
Sudden onset testicular pain and swelling; occasionally nausea, vomiting. Note: pain may be in the iliac fossa |
Discolouration of scrotum; exquisitely tender testis, riding high |
|
Torsion of the appendix testis (hydatid of Morgagni) |
More gradual onset of testicular pain |
Focal tenderness at upper pole of testis; "blue dot" sign – necrotic appendix seen through scrotal skin Note: Difficult to distinguish from testicular torsion |
|
Epididymoorchitis |
Onset may be insidious; fever, vomiting, urinary symptoms; rare in pre-pubertal boys, unless underlying genitourinary anomaly, when associated with UTI. |
Red, tender, swollen hemiscrotum; tenderness most marked posteriolateral to testis. Pyuria may be present. |
|
Incarcerated inguinal hernia |
History of intermittent inguinoscrotal bulge, with associated irritability |
Firm, tender, irreducible, inguinoscrotal swelling |
|
Idiopathic scrotal oedema |
Swelling noted but child not distressed |
Bland violaceous oedema of scrotum, extending into perineum + penis; testes not tender |
|
Hydrocele |
Swollen hemiscrotum in well, settled baby |
Soft, non-tender swelling adjacent to testis; transilluminates brightly. |
|
Varicocele |
Collection of abnormally enlarged spermatic cord veins, found in teenage boys, mostly on the left. |
Mass of varicose veins ("bag of worms") above testicle, non-tender, more prominent when standing. |
|
Henoch Schonlein purpura |
Painful scrotal oedema, with purpuric rash over scrotum. May have associated vasculitic rash of buttocks and lower limbs, arthritis, abdominal pain with GI bleeding, and nephritis |
may be difficult to distinguish from testicular torsion in absence of other features |
|
Testicular or epididymis rupture |
Scrotal trauma eg. straddle injury, bicycle handlebars, sports injury. Delayed onset of scrotal pain and swelling. |
Tender swollen testis. Bruising, oedema, haematoma, or haematocele may be present. |
|
Testis tumor |
Can be painful in rapid growing tumors associated with haemorrhage or infarction |
Painless, unilateral, firm to hard scrotal swelling. Leukaemic infiltration may present bilaterally. |
|
Antenatal torsion testis |
Newborn may present with painless, smooth, testicular enlargement. |
Does not transilluminate, dark in colour. |
In the early phase, location of the pain can lead to the diagnosis. Patients with acute epididymitis experience a tender epididymitis, while patients with testicular torsion are more likely to have a tender testicle and patients with torsion of the appendix testis feel isolated tenderness of the superior pole of the testis.
An abnormal position of the testis was more frequent in testicular torsion than in patients with epididymitis.
Looking for the absence of the cremasteric reflex is a simple method with a sensitivity of 100% and specificity of 66% for the presence of testicular torsion [14, 43].
The classical sign of a 'blue dot' (this is the appendix of the testis which has become discolored and is noticeably blue through the skin) was found only in 10-25% patients with torsion of the appendix testis [39, 36].
Fever occurs often in epididymitis - 11-19%.
A positive urine culture is only found in a few patients with epididymitis. It should be remembered that a normal urinalysis does not exclude epididymitis. Similarly, an abnormal urinalysis does not exclude testicular torsion.
The use of Doppler ultrasound may reduce the number of patients with acute scrotum undergoing scrotal exploration. It may also show a misleading arterial flow in the early phases of torsion and in partial or intermittent torsion: persistent arterial flow does not exclude testicular torsion.
Doppler ultrasound is a highly effective imaging tool to evaluate acute scrotum and comparable to scintigraphy and dynamic contrast-enhanced subtraction MRI [30].
A discussion of the most important and common conditions that cause acute scrotal pain or swelling follows:
Testicular Torsion
Torsion of the testicle results from twisting of the spermatic cord, which compromises testicular blood supply.
Testicular torsion is a true surgical emergency.
Torsion of the testis occurs most often in the neonatal period and around puberty [3, 8, 30]. The true incidence of testicular torsion is unknown [14].
Torsion more often involves the left testicle.
Classification
|
The types of testicular torsion: |
|
|
* Extravaginal torsion (4% of all torsion patients [14]) results from twisting of the cord proximal to the tunica vaginalis. This mechanism occurs perinatally during descent of the testicle before the scrotal investment of the tunica vaginalis has taken place, allowing the tunica and testis to spin on their vascular pedicles. The tunica vaginalis likely becomes adherent to the surrounding tissues by 6 weeks of age.
In newborns, torsion is classically described as “extravaginal” because the tunica vaginalis is only weakly attached to the overlying dartos muscle (A). After 6 to 8 weeks of extrauterine life, these attachments become much stronger and extravaginal torsion is exceedingly uncommon.
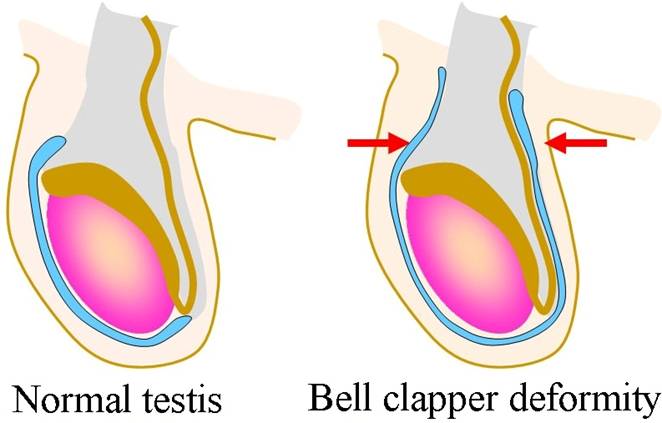
* Intravaginal torsion (96% of all torsion patients [14]) occurs beyond the perinatal period and can result from abnormal fixation of the testicle and epididymis within the tunica vaginalis which is described classically as the “bell-clapper” deformity and occurs in only a minority of males (Image 8.1). Because testicular torsion occurs relatively infrequently, other factors also play a role in its occurrence.
Torsion of the cord in an older child is classically described as “intravaginal” (B) or because the mesorchium is somewhat longer than normal (C).
|
|
|
Image 8.1 “Bell-clapper” deformity, a congenital condition in which the testis hangs within the scrotum (red arrows) and can swing like a bell clapper in a bell, allowing for easy torsion. Males born with the bell clapper deformity have no attachments around either testicle, so that torsion can potentially occur on either side. The bell clapper deformity is present in approximately 12% of males; 40% of them are affected in both testicles. |
Etiology
The etiologic factors involved in intravaginal testicular torsion include congenital anomaly, bell clapper deformity, undescended testicle, sexual arousal or activity, exercise, active cremasteric reflex, and cold weather [3, 29, 36, 43].
Rapid growth and increasing vascularity of the testicle also may be precursors to torsion. This phenomenon occurs at puberty and is believed to account for the age distribution of torsion, which increases in adolescence.
Rapid cremasteric muscle contraction elevates the testicle and can have a rotational effect on the spermatic cord that can induce torsion.
Clinical Presentation
The sudden onset of severe unilateral pain, often with nausea and vomiting, is the classic presentation of testicular torsion.
The abdomen and inguinal area should be inspected for other causes of scrotal pain, such as an inguinal hernia.
Depending on the duration of the torsion, the scrotum can show various degrees of erythema and induration.
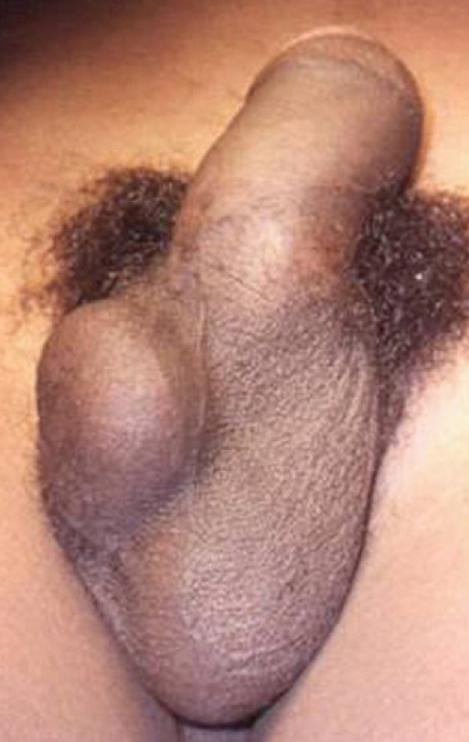
The involved testicle may be riding higher (the involved testicle is "high-riding"(Image 8.2)), have a transverse orientation, or have the epididymis located anteriorly.
|
|
|
Image 8.2 High riding right testis. |
Neonates with unilateral cryptorchidism may have had an in utero torsion. Prenatal testicular torsion will present with an empty scrotum. Others may present with a red, firm, tender mass of the groin area which may not be distinguishable from an incarcerated hernia.
Intrauterine torsion may present as: testicular nubbin; small and hard testis; normal-sized and hard testis [30].
The cremasteric reflex frequently is absent, but presence of the cremasteric reflex certainly does not exclude torsion [29, 36].
NB: The cremasteric reflex – stimulation of the skin on the front and inner thigh (over Scarpa's triangle) retracts the testis on the same side. Stimulus usually causes cremasteric muscle contraction.
- Normal: Cremasteric reflex present (testicle rises). Seen in Epididymitis;
- Abnormal: Cremasteric reflex absent (no testicle rise). Suggests Testicular Torsion
Also absent in 50% of boys under age 30 months (do not use this test under age 30 months!).
In boys, this reflex may be exaggerated which can occasionally lead to a misdiagnosis of cryptorchidism.
The cremasteric reflex can be helpful in recognizing testicular emergencies. The presence of the cremasteric reflex does not eliminate testicular torsion from a differential diagnosis, but it does broaden the possibilities to include epididymitis or other causes of scrotal and testicular pain.
Imaging
High-resolution ultrasonography with color-flow Doppler is better for direct visualisation of spermatic cord twisting [29, 30, 36]. This studies provide information about testicular structure and their blood flow.
Scintigraphy and, more recently, dynamic contrast-enhanced subtraction MRI of the scrotum also provide a comparable sensitivity and specificity to ultrasound. These investigations may be used when diagnosis is less likely and if torsion of the testis still cannot be excluded from history and physical examination. This should be done without inordinate delays for emergency intervention [30].
Treatment
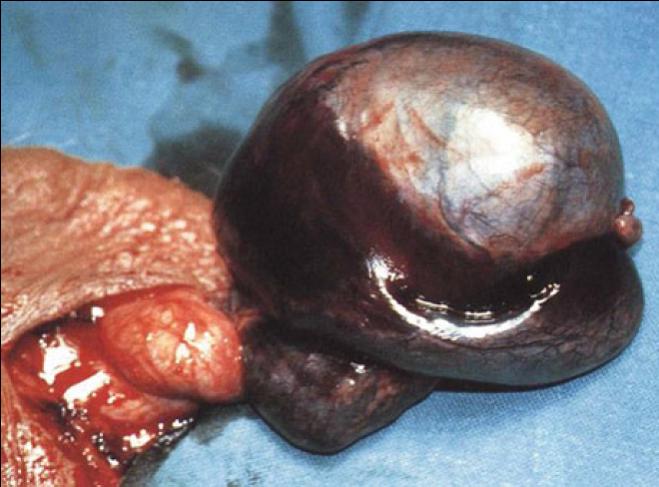
Testicular torsion is an urgent condition, which requires prompt surgical treatment as quickly as possible within the limits of surgical and anesthetic safety. Time wasted attempting to arrange for imaging studies, laboratory testing, or other diagnostic procedures results in lost testicular tissue (Image 8.3).
|
|
|
Image 8.3 Necrotic testicle secondary to testicular torsion. |
The two most important determinants of early salvage rate of the testis are the time between onset of symptoms and detorsion, and the degree of cord twisting [30].
Manual detorsion
According to the guidelines 2014 of the European Society for Paediatric Urology (ESPU) in some cases of testicular torsion, manually untwisting the spermatic cord may allow reestablishment of vascular flow (Level of evidence: 3; Grade of recommendation: C) [29, 30, 36].
If a patient presents early in the course of testicular torsion (less than 12 h), manual detorsion should be attempted. It should initially be done by outwards rotation of the testis unless the pain increases or if there is obvious resistance [30, 36].
Technique: Manual detorsion of the testis is done without anaesthesia [30]. The technique involves manipulating the involved testis so that the anterior surface rotates from medial to lateral. This is termed the "open book" method because the motion resembles opening the cover of a book. However testicular torsion does not always occur in a uniform direction. Success is defined as the immediate relief of all symptoms and normal findings at physical examination.
Manual detorsion is best performed with the intention of buying time until the surgical team is ready, rather than with the intention of altogether avoiding a surgical procedure. It may play a role in decreasing the degree of ischemia when a substantial delay in reaching the operating room is anticipated, but it is not a substitute for exploration and fixation [29, 36].
NB: However, application of this maneuver in a child with a swollen painful scrotum can be difficult or impossible without anesthesia. In addition, knowing which way the testis is torsed a priori is impossible; thus, attempting detorsion may simply worsen the degree of torsion. In actuality, manual detorsion is difficult and rarely used.
Surgical Exploration
When testicular torsion cannot be excluded, exploration is warranted!
Urgent surgical exploration is mandatory in all cases of testicular torsion within 24 hours of the onset of symptoms. In patients with testicular torsion of more than 24 hours, semi-elective exploration is necessary (Level of evidence: 3; Grade of recommendation: C) [30].
Exploration can involve paramedian scrotal incision, transverse incision, or single midline scrotal incision. Some surgeons prefer to explore the acute scrotum through an inguinal incision, based on the theory that this approach offers better control of the high spermatic cord if the exploration reveals an unexpected diagnosis (eg, testis tumor, incarcerated hernia).
It may be difficult to intraoperatively determine whether a testis of marginal viability should be retained or excised. The affected testicle is inspected, detorsed, and placed in a warm sponge. Up to 30 minutes observation is acceptable.
If doubt remains regarding viability, the testis is incised to determine the presence of bleeding. The flow of the tunica may be restored to some extent while the parenchyma remains underperfused. Debate ranges regarding the treatment of marginally viable testicle. If atrophy or hypoplasia ensue, the testicle is removed.
It is controversial whether testes of doubtful viability should be excised or left in situ to see whether they will recover any hormonal function.
There is now good evidence that testicular ischemia damages the blood-testis barrier and exposes the child older than 10 years of age to the potential risk of autoimmunization (formation of antisperm antibodies; this may affect sperm produced by both testes) against his own spermatogonia. Both spermatogenesis and the blood-testis barrier are established after age 10 years; thus, some surgeons always retain the doubtful testis in children younger than 10 years, in older patients - orchidectomy [36, 43].
NB: The risk of autoimmunization related to ischemia is low in children younger than age 10 because there is no blood-testis barrier before spermatogenesis commences.
There is no common recommendation about the preferred type of fixation and suture material; however, many urologists currently use a Dartos pouch orchiopexy (2-3 sutures are passed through the dartos and tunica albuginea of the testicle). Some surgeons avoid placing sutures directly into the tunica albuginea out of concern for disrupting the blood-testis barrier; instead, they place the sutures into the visceral tunica vaginalis of the mesorchium [29, 30, 36, 43].
Although torsion of the contralateral testis is extremely rare, many clinicians, fueled by fear of litigation, have become more aggressive with surgical exploration to fix the contralateral side and prevent future torsion. This is an area of considerable controversy!
Notwithstanding according to the current guidelines of the European Society for Paediatric Urology if torsion is confirmed, contralateral orchiopexy is recommended. This should not be done as an elective procedure, but rather immediately following detorsion [30].
Recurrence after orchiopexy is rare and may occur several years after operation.
Outcomes
Outcomes directly depend on the duration of ischemia; thus, time is of the essence.
For torsion less than 6 hours, 85-97% can be salvaged. If the duration of torsion exceeds 24 hours, the chance of salvaging the testes is less than 10% [14].
Prognosis
Fertility: The results vary and are conflicting.
Androgen levels: Endocrine testicular function remains in the normal range in patients after testicular torsion [30].
Testicular cancer: There may be a 3.2-fold increased risk of developing a testis tumour 6-13 years after torsion [30].
Perinatal Testicular Tortion
Perinatal torsion is a term used to include both prenatal and postnatal events. Most cases are extravaginal torsion in contrast to the usual intravaginal torsion, which occurs during puberty.
The difference between the two types of torsion is important but sometimes may be difficult to determine clinically.
¤ Prenatal torsion classically presents at birth as a hard, nontender mass in the hemiscrotum, usually with underlying dark discoloration of the skin and fixation of the skin to the mass. This picture is characteristic of infarction of the testis caused by previously occurring torsion.
¤ Postnatal torsion presents with more classic, acute inflammation, including erythema and tenderness. A report of a previously normal scrotum at delivery suggests an acute event.
In the neonate with torsion at birth or a few days afterward, surgical recommendations are controversial. Although most authorities recommend exploration of the ipsilateral side and fixation of the contralateral testis (because of the risk of asynchronous contralateral testicular torsion in as many as 33% of cases [30]), some have suggested that observation is acceptable because of the negligible salvage rate of the ischemic testis and the low incidence of contralateral torsion. The risk of anesthesia in children younger than 1 year may also factor into decision-making process.
According to the current guidelines of the ESPU the contralateral scrotum should be explored [30].
Extravaginal testicular torsion that occurs in newborns cannot be treated by manual detorsion.
Exploration in the neonate should be by inguinal incision because torsion may occur within the canal [45].
Torsion of the appendix testis (testicular appendages or hydatid of Morgagni)
Torsion of testicular appendages can result in the clinical presentation of acute scrotum.
Two such appendages are the appendix testis, a vestigial embryologic remnant of the paramesonephric (müllerian) duct, and the appendix epididymis, a vestigial embryologic remnant of the mesonephric (wolffian) duct [45].
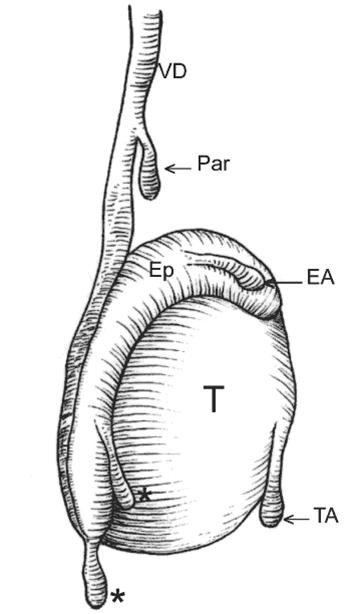
The appendix testis is present in 92% of all testes and is usually located at the superior testicular pole in the groove between the testicle and the epididymis. The appendix epididymis is present in 23% of testes and usually projects from the head of the epididymis, but its location may vary (Image 8.4).
|
|
Image 8.4 Testis and epididymal appendages. TA – testicular appendage (appendix testis); EA – epididymal appendage (appendix epididymis); Par – paradidymis – organ of Giraldes; Asterisks – superior and inferior vas aberrans of Haller; T – testis; Ep – epididymis; VD – vas deferens. |
Age ranges vary from infancy to adulthood with more than 80% of cases occurring in children aged 7-14 years. Mean age is 10.6 years. This condition rarely presents in adulthood (probably due to local fibrosis). Torsion of testicular appendices is the leading cause of acute scrotum in children.
Pathophysiology
The vestigial tissues forming the appendices are commonly pedunculated and are structurally predisposed to torsion. Torsion of an appendage leads to ischemia and infarction. Necrosis of appendices causes pain and local inflammation of surrounding the tunica vaginalis and epididymis (acute hemiscrotum). Torsion of the testicular appendage may also be accompanied by presence of a thickened scrotal wall, a reactive hydrocele, and enlargement of the head of the epididymis.
Presentation
The patient's history is important in distinguishing torsion of the testicular appendages from testicular torsion and other causes of acute scrotum.
· Pain may be present.
* Onset is usually acute, but pain may develop over time. Typically, it has a more gradual onset than testicular torsion.
* Intensity ranges from mild to severe.
* Patients may endure pain for several days before seeking medical attention.
* The pain is located in the superior pole of the testicle. This is a key distinguishing factor from testicular torsion. A focal point of pain on the testicle is uncommon in complete testicular torsion.
· Systemic symptoms are absent. Nausea and vomiting (frequently seen in testicular torsion) are usually not associated with this condition.
· Urinary symptoms are absent. Dysuria and pyuria are not associated with torsion of the testicular appendages. Their presence is more indicative of epididymitis.
Physical examination may reveal the following findings:
§ The patient is afebrile with normal vital signs.
§ Although the scrotum may be erythematous and edematous, it usually appears normal.
§ An unreliable marker of pathology, the cremasteric reflex is usually intact. Several studies indicate that the presence of a cremasteric reflex in the acute scrotum is unlikely to be testicular torsion.
§ The testis should be nontender to palpation. If present, tenderness is localized to the upper pole of the testis. Diffuse tenderness is more common in testicular torsion.
§ The presence of a paratesticular nodule at the superior aspect of the testicle, with its characteristic blue-dot appearance, is pathognomonic for this condition. A blue-dot sign is present in only 21% of cases.
§ The combination of a blue-dot sign with clear palpation of an underlying normal, nontender testes allows for the exclusion of testicular torsion on clinical grounds alone.
§ Vertical orientation of the testes is preserved.
The differential diagnosis includes testicular torsion, epididymitis, Henoch-Schonlein Purpura, hernias, hydrocele, orchitis.
Imaging Studies
- High-resolution ultrasonography with color-flow Doppler
- Radionuclide imaging (Scintigraphy)
NB: Scintigraphy and, more recently, dynamic contrast-enhanced subtraction MRI of the scrotum also provide a comparable sensitivity and specificity to ultrasound. These investigations may be used when diagnosis is less likely [30].
|
|
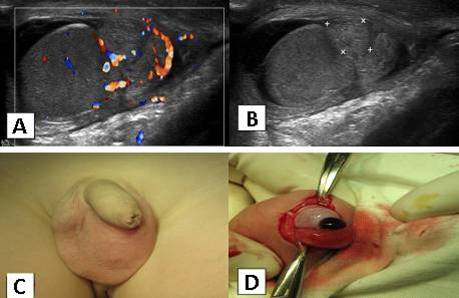
|
Image 8.5 Torsion of testicular appendages of the left testis. A – Color Doppler sonography; B – Ultrasonography; C – Left scrotum is erythematous and edematous, D – Intraoperative photo demonstrating the torsion of testicular appendages. |
Treatment
§ Necrotic tissue of the testicular appendices causes no damage other than damage to itself. Most cases, therefore, are treated conservatively.
§ Pain usually resolves within 1 week but may persist for several weeks.
§ Antibiotics and NSAIDs are the mainstays of therapy for inflammation.
§ Reduced activity and scrotal support (e.g. tight underwear or jock strap) are indicated.
§ Uncontrolled pain can be relieved by surgical excision of the appendix.
NB: According to the guidelines 2014 of the ESPU and the American Association of Pediatric Urologists (AAPU) torsion of the appendix testis can be managed conservatively (Level of evidence: 4; Grade of recommendation: C) but in equivocal cases and in patients with persistent pain, surgical exploration is indicated [30].
Epididymo-orchitis
Epididymitis means inflammation of the epididymis. Orchitis means inflammation of a testis.
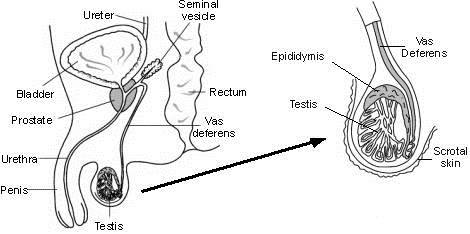
As the epididymis and testis lie next to each other, it is often difficult to tell if the epididymis, the testis, or both are inflamed. Therefore the term epididymo-orchitis is often used (Image 8.6).
|
|
|
Image 8.6 Schema of epididymo-orchitis anatomy. |
Epididymitis is the most common inflammatory process involving the scrotum and more common in adults.
Etiology
In prepubertal boys, the etiology is usually unclear, with an underlying pathology of about 25%. A urine culture is usually negative, and unlike in older boys, a sexually transmitted disease is very rare [30].
Infections generally originate in the lower urinary tract from the bladder, urethra or prostate and are typically caused by urinary tract pathogens (e.g. E. coli, Streptococcus or Staphylococcus) or sexually transmitted organisms (Chlamydia or gonorrrhhea).
A sterile chemical epididymitis can result from reflux of sterile urine through the ejaculatory ducts, for instance if the ureter inserts in the prostatic urethra, this may lead to increased pressure in the vas deferens.
Diagnosis
In the early phase, location of the pain can lead to diagnosis. Patients with acute epididymitis experience a tender epididymis.
Orchitis is characterized by focal, peripheral, hypoechoic testicular lesions that are poorly defined, amorphous, or crescent-shaped.
Fever occurs often in epididymitis (11-19%) [30].
A reactive hydrocele is also frequently associated with epididymoorchitis.
A positive urine culture is only found in a few patients with epididymitis. It should be remembered that a normal urinalysis does not exclude epididymitis.
The use of Doppler ultrasound may reduce the number of patients with acute scrotum undergoing scrotal exploration, but it is operator-dependent and can be difficult to perform in prepubertal patients [30, 43].
Treatment
Antibiotic treatment, although often started, is not indicated in most cases unless urinalysis and urine culture show a bacterial infection. Epididymitis is usually self-limiting and with supportive therapy (i.e. minimal physical activity and analgesics) heals without any sequelae (Level of evidence: 3; Grade of recommendation: C) [30].
However, bacterial epididymitis can be complicated by abscess or necrotic testis and surgical exploration is required.
Treatment is directed toward the etiologic cause [29]:
· If the epididymo-orchitis is idiopathic, then treatment is similar to treatment for torsion of the appendix testis e.g., NSAIDs, scrotal support and limited activity for 7 days.
· If the etiology is infectious then treatment includes empiric antibiotics until definitive cultures with sensitivity results are available that confirm the empiric treatment or direct new and different antibiotics.
· If there is suspicion that the patient has a sexually transmitted disease, the adolescent is treated with Ceftriaxone plus Doxycycline.
· For acute epididymitis most likely caused by enteric organisms or with negative gonococcal culture or nucleic acid amplification test, treatment is Ofloxacin or Levofloxacin.
Disorders of the penis and foreskin
Phimosis
Phimosis is defined as the inability to retract the foreskin.
At birth, physiologic phimosis is present as adhesions between the prepuce and glans preclude retracting the foreskin. As the child grows, the two layers begin to separate as sloughed epithelial debris, or smegma, accumulates between them, defining this plane. This smegma is commonly referred to as "foreskin pearls" and can be mistaken for infection or purulence by the uneducated parent.
Classification
The phimosis is either primary (physiological) with no sign of scarring, or secondary (pathological) to a scarring such as balanitis xerotica obliterans.
Some authors use the term ''non-retractile foreskin" to distinguish this developmental condition from pathologic phimosis.
NB: Pathologic phimosis has to be distinguished from normal agglutination of the foreskin to the glans, which is a physiological phenomenon.
Incidence
Most uncircumcised infants have normal, physiologic phimosis (non-retractile foreskin). At the end of the first year of life, retraction of the foreskin behind the glandular sulcus is possible (with spontaneous erections and natural manipulation) in only about 50% of boys; this rises to approximately 90% by the age of 3 years. The incidence of phimosis is 8% in 6 to 7-year-olds and just 1% in males aged 16-18 years [30].
Diagnosis
The diagnosis of phimosis is made by physical examination. There may be a history of ballooning of the foreskin during urination, with dribbling of entrapped urine after voiding is complete.
Forceful retraction is not required for this to occur and may initiate the vicious cycle of tearing and scarring, which can lead to pathologic phimosis.
Treatment
- In children older than 4 years who are unable to retract the foreskin and are symptomatic with episodes of posthitis or balanoposthitis, recurrent UTI or ballooning of the foreskin with voiding, a trial of betamethasone cream (0.05%) two times per day for 1 to 2 months allows the foreskin to retract in up to 90% of boys [29, 30, 43].
This treatment has no side effects and the mean blood spot cortisol levels are not significantly different from an untreated group of patients [43].
Surgery
An absolute indication for surgery is secondary phimosis.
In primary phimosis recurrent balanoposthitis and recurrent urinary tract infections in patients with urinary tract abnormalities are indications for intervention (Level of evidence: 2b; Grade of recommendation: B) [30]. For those refractory to corticosteroid treatment, the surgery is indicated [43].
Surgical methods range from the complete removal of the foreskin to more minor operations to relieve foreskin tightness:
- temporizing dorsal slit (dorsal incision)
- preputioplasty (surgical enlargement of the phimotic ring)
- circumcision
Paraphimosis
The most acute complication of phimosis is paraphimosis. Paraphimosis is the term used to describe the condition that occurs when a narrow foreskin is forcibly retracted and becomes trapped behind the head of the penis [2, 3, 8].
The retracted foreskin initially blocks lymphatic drainage from the distal penis, progressively causing further edema of the retracted foreskin. If the foreskin remains retracted and the edema continuous, venous obstruction followed by arterial flow are expected within hours to days.
The foreskin does not become fully mobile before the age of 3-4 years, predisposing children younger than 3-4 years to paraphimosis when their caregivers retract the foreskin for cleaning.
Clinical presentation
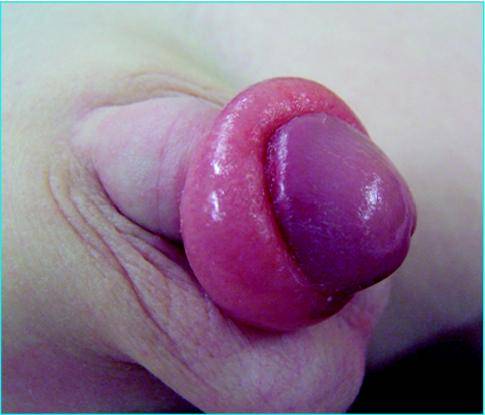
Patients present with a red, painful, and swollen glans penis associated with an edematous, proximally retracted foreskin that forms a circumferential constricting band (Image 8.7).
|
|
Image 8.7 The paraphimosis is characterized by retracted foreskin with the constrictive ring localized at the level of the sulcus, which prevents replacement of the foreskin over the glans. |
Pain and swelling make it difficult to return the foreskin to the non-retracted position.
NB: Forcible retraction of a narrow foreskin should be avoided. The foreskin should be returned to the forward position after cleaning or sexual intercourse.
The diagnosis of paraphimosis is made by physical examination.
Treatment
Paraphimosis should be treated as a medical emergency, as it can result in gangrene or other serious complications (a risk of consecutive necrosis).
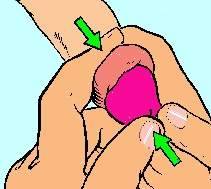
Treatment of paraphimosis consists of manual reduction (compression) of the oedematous tissue with a subsequent attempt to retract the tightened foreskin over the glans penis.
Manual reduction
The swollen area is gently but firmly compressed within one hand, for a few minutes, to squeeze out the oedema fluid. Manual reduction is performed by placing both index fingers on the dorsal border of the penis behind the retracted prepuce and both thumbs on the end of the glans. The glans is pushed back through the prepuce with the help of constant thumb pressure while the index fingers pull the prepuce over the glans (Image 8.8).
|
|
Image 8.8 Manual reduction.
|
NB: Paraphimosis can usually be corrected without surgery.
This technique may be facilitated by the use of ice and/or hand compression on the foreskin, glans, and penis to minimize edema of the glans prior to manual reduction.
Soaking the penis in a glove full of ice for 5 minutes before attempting manual reduction has been reported to be effective 90% of the time (Kessler C.S., Bauml J., 2009).
Adequate analgesia or sedation should be given. Liberally covering the entire foreskin and glans in topical anaesthetic cream for 1 hour may be effective. Local infiltration of anaesthetic is best avoided as it increases the swelling.
When the foreskin has been returned to its normal position, no further treatment is necessary.
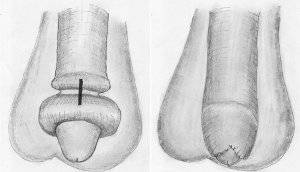
If manual reduction manoeuvre fails, a dorsal incision of the constrictive ring is required (Image 8.9) [3, 8]. Depending on the local findings, a circumcision is carried out immediately or can be performed in a second session [30].
|
Image 8.9 The dorsal incision cuts the phimotic ring in longitudinal direction. After the incision, the prepuce should be retractable without resistance. Transverse suture of the dorsal incision closes the skin defect and helps in hemostasis. Circumcision should be postponed until the edema of the prepuce has resolved. |
Balanitis
Balanitis is inflammation of the glans penis. If the foreskin is also inflamed, the correct term is balanoposthitis, although balanitis is commonly used to refer to both.
Definitions:
- · balanitis – inflammation of the glans penis
- · posthitis – inflammation of the foreskin
- · balanoposthitis – inflammation of the glans penis and foreskin
Inadequate hygiene is the most common cause of nonspecific acute balanoposthitis, which usually occurs in boys aged 2 to 5 years. Irritation from soaps, bubble baths, laundry detergent, and antistatic sheets have also been implicated. Balanitis in children usually arises from an infection of the smegma on the basis of a phimosis or non-retractile foreskin.
Other causes include trauma from masturbation and zip-fastener injuries.
Risk factors:
* The most important risk factor is diabetes mellitus.
* Poor hygiene in uncircumcised males.
* Immunosuppression.
* Chemical or physical irritation of glans.
The organisms most commonly involved are faecal bacteria and candida. Streptococcus pyogenes, Staphylococcus aureus are the usual bacterial pathogens in children. Viral and protozoal infections are reported causes in third world countries.
Balanitis xerotica obliterans is a rare cause of acute balanoposthitis in children. This condition manifests as whitish plaques on the surface of glans and prepuce, usually around the corona and up to the external meatus; the foreskin is thickened, fibrous, and nonretractable. Atrophic white patches appear on the affected area, and commonly, a whitish ring of indurated (hardened) tissue usually forms near the tip that may prevent retraction.
Balanitis xerotica obliterans and rarer skin conditions require referral to an urologist or dermatologist [30].
Clinical features of nonspecific acute balanoposthitis include pain, erythema, and swelling of the glans penis and prepuce. In most cases, there is little or no discharge. True urethral discharge, suggestive of sexually transmitted disease, is seen after milking the length of the urethra starting from the base of the penis.
Investigations
* Blood/urine testing for glucose if diabetes mellitus is possible.
* Swab of discharge for microscopy, Gram staining, culture and sensitivity.
Treatment
Treatment depends on the underlying cause.
Treatment of nonspecific acute balanoposthitis usually includes:
- Local hygiene (keep area clean by bathing 2 to 3 times a day while symptoms persist)
- Warm bath with dilute saline
- Warm bath with potassium permanganate solution (1 in 10,000 dilution) is nearly always beneficial when used to wash the penis but causes temporary purple discolouration
+/-
- In most cases topical treatment is recommended.
- Topical hydrocortisone 1% cream (twice a day) or ointment may help in mild cases (steroid creams of mild to moderate strength are used in short-term courses for non-infective eczematous or inflammatory skin conditions).
- Topical antibiotics creams are sometimes used but are of unproven efficacy.
- If candidal infection is the suspected cause: clotrimazole cream 1% or miconazole cream 2%; apply twice daily until symptoms have settled.
+/-
- Systemic therapy should be considered if there is severe inflammation affecting the penile shaft, or marked genital oedema.
- If there is significant bacterial infection of the whole of the foreskin or the skin of the penile shaft then bacterial infection is likely and antibiotics should be given. Most cases respond to oral antibiotics. Occasional cases require admission for parenteral antibiotics.
· Analgesia is important, and sitting in a warm bath may ease dysuria.
- Surgical referral for consideration of circumcision if balanitis is recurrent or pathological phimosis is present [30, 43].
In children, symptoms typically resolve 3 to 5 days after treatment is started.
Recurrent balanitis may cause a phimosis with disturbance of micturition.
Genitourinary Tract Trauma
The genitourinary tract is involved only in 3% of pediatric trauma cases. However, the management of these injuries can be challenging.
Renal Trauma
Incidence
Almost 50% of genitourinary tract injuries involve the kidney. 90 % of renal injuries in children are due to blunt force trauma and approximately 10% of pediatric blunt abdominal trauma causes injury to the kidney [14, 30, 43]. Renal injury occurs in about 3% to 6% of patients with penetrating trauma [29, 43].
Renal trauma is caused by either blunt injuries from falls, car accidents, sports injuries, physical assault, and sexual abuse, or penetrating injuries, usually due to falls onto sharp objects or from gunshot or knife wounds.
Children are more likely than adults to sustain renal injuries after blunt trauma because of their anatomy. Compared to an adult kidney, a child's kidney is larger in relation to the rest of the body and often retained foetal lobulations, so that blunt trauma is more likely to lead to a local parenchymal disruption. The paediatric kidney is also less well protected than the adult kidney. Children have less perirenal fat, much weaker abdominal muscles, and a less ossified and therefore much more elastic and compressible thoracic cage [14, 29, 30].
An associated abnormality, congenital (ureteropelvic junction obstruction, ectopic kidney) or otherwise (Wilms' tumor), makes it even more susceptible to injury.
Clinucal Presentation
Patients present with flank pain and hematuria (microscopic or gross). Abdominal tenderness, flank mass, flank hematoma and fractured ribs are important signs of renal trauma [6, 8].
Classification
In 1989, the American Association for the Surgery of Trauma (AAST) Organ Injury Scaling Committee devised and published a classification or grading system for genitourinary tract injuries (Table 8.5) to standardize injury descriptions for research and data collection purposes.
For the kidney, this grading system has proved highly applicable, and its usefulness as a measure of the seriousness of renal injury and as a predictor of clinical outcomes.
For example, patients with a grade I injury require observation only, whereas those with a grade V injury are more likely to require nephrectomy. Those with intermediate injuries (grades II to IV) require individualized therapy, with a trend toward more invasive therapy as injury grade increases.
Table 8.5
|
Renal Injury Scale of the AAST |
||
|
Grade |
Type of injury |
Description |
|
I |
Contusion |
Microscopic or gross haematuria |
|
Haematoma |
Normal urological studies |
|
|
II |
Haematoma |
Non-expanding subcapsular haematoma |
|
Laceration |
Laceration of the cortex of less than 1.0 cm |
|
|
III |
Laceration |
Laceration > 1.0 cm without rupture of collecting system |
|
IV |
Laceration |
Through the cortex, medulla and collecting system |
|
Vascular |
Vascular injury |
|
|
V |
Laceration |
Completely shattered kidney |
|
Vascular |
Avulsion of the renal hilum |
|
Diagnosis
In a child who has sustained blunt abdominal trauma, renal involvement can often be predicted from the history, physical examination and laboratory evaluation [2, 6, 7].
It is important to consider that children, unlike adults, are able to maintain their blood pressure, even in the presence of hypovolemia, due to compliance of the vascular tree and mechanisms for cardiac compensation [29, 30, 36].
Renal involvement may be associated with abdominal or flank tenderness, lower rib fractures, fractures of vertebral pedicles, trunk contusions and abrasions, and haematuria [43].
Laboratory
The degree of hematuria does not always correlate with the severity of the injury and children with renal pedicle injuries and/or pedicle disruptions may present without hematuria [43].
However haematuria may be a reliable finding. In severe renal injuries, 65% suffer gross haematuria and 33% microhaematuria, while only 2% have no haematuria at all [30].
Imaging
Imaging is recommended in all children who have sustained a blunt or penetrating trauma with any level of haematuria, especially when the history reveals a deceleration trauma, direct flank trauma or a fall from a height.
¤ CT - the most sensitive and specific test to evaluate renal trauma is CT with intravenous contrast.
¤ IVP - if the patient is unstable or requires immediate surgery, a "one shot" intravenous pyelogram is performed by administering a 2 mL/kg bolus of radiographic contrast and obtaining a single supine radiograph of the abdomen 10 minutes later [14].
¤ Ultrasound - in acute trauma ultrasound may be used as a screening tool and for reliably following the course of renal injury [3, 30].
NB: Ultrasound is of limited value in the initial and acute evaluation of trauma. The standard IVP is a good alternative imaging method if a CT scan is not available. It is superior to ultrasound but not as good as CT scanning for diagnostic purposes [30].
Treatment
The modern management of trauma is multidisciplinary, requiring paediatricians, surgeons, urologists, and other specialties as required.
Most injured kidneys can be managed conservatively (Grade of recommendation: B) [30].
Blunt Injuries:
Non-surgical conservative management with bed rest, fluids and monitoring has become the standard approach for treating blunt renal trauma [2, 8, 29, 30, 36].
Even in high-grade renal injuries, a conservative approach is effective and recommended for stable children. However, this approach requires close clinical observation, serial CT scans, and frequent re-assessment of the patient's overall condition [14, 30, 43].
Absolute indications for surgery include haemodynamic instability and a Grade V renal injury (Grade of recommendation: A). Relative indications for surgery are massive urinary extravasation and extensive non-viable renal tissue [30].
Penetrating Injuries:
Penetrating renal injuries are rare in children. Although there is a role for selective nonoperative management, most wounds require surgical exploration [3, 8, 14].
Selection criteria for nonoperative management include hemodynamic stability, CT for grading of kidney injury and excluding other associated injuries requiring exploration [14].
However, a high index of suspicion for missed ureteral and other associated injuries must be maintained if a nonoperative pathway is chosen.
Obviously, severe renal injuries require operative intervention consisting of drainage, repair, or nephrectomy.
Complications
Although most renal injuries in children can be managed nonoperatively, this type of management is not without complications.
|
Short-term |
Long-term |
|
|
|
|
|
|
These complications are generally seen after injuries in which segments of parenchyma are devascularized or extensive hemorrhage and urinary extravasation have occurred.
Ureteral Trauma
Ureteral injuries uncommon, accounting for less than 1% of all genitourinary traumas [30, 43].
The overwhelming majority of ureteral injuries is due to penetrating trauma from gunshot wounds (94%) or stab wounds (2.5%), with blunt trauma accounting for only 3% of all ureteral injuries [43].
The ureter is well protected; the upper part is protected by its close approximation to the vertebral column and paraspinal muscles and the lower part by its route through the bony pelvis. In addition, the ureter is a small target, and both flexible and mobile [14, 30].
The ureteral injuries are caused more often by penetrating trauma than blunt trauma.
Diagnosis
There are no classical clinical symptoms suggestive of ureteral trauma. Because the symptoms may often be quite vague, it is important to remain suspicious for a potential undiagnosed urinary injury following significant blunt abdominal trauma in a child [30, 43].
Hematuria is rarely seen with ureteric injuries. Quite a few patients present several days after the injury, when the urinoma produces flank and abdominal pain, nausea and fever [14].
Unfortunately, initial imaging studies, such as IVP and routine CT scans, are unreliable [30].
The ureteral injuries are better evaluated by an abdominal CT scan with intravenous contrast (delayed CT scan up to 10 minutes after injection of the contrast) [14].
But the most sensitive diagnostic test is a retrograde pyelogram (Grade of recommendation: A) [30].
NB: Retrograde pyelogram is the most sensitive diagnostic method and is the method of choice. However, in the initial phase of an injury, it is very likely that ureteral injuries will not be detected by routine imaging methods, including contrast-enhanced spiral CT [30].
Management
Traumatic avulsions are best repaired immediately. Partial tears are usually repaired, but they could be managed nonoperatively [14].
Endoscopic treatment is the method of choice, such as internal stenting or drainage of a urinoma, either percutaneously or via a nephrostomy tube [30].
If endoscopic management is not possible, primary repair of partial lacerations should be followed by internal stenting. The management of complete lacerations, avulsions or crush injuries depends on the amount of ureter lost and its location. If there is an adequate healthy length of ureter, a primary ureteroureterostomy can be performed. If primary re-anastomosis is not achievable, distal ureteral injuries can be managed using a psoas bladder hitch, Boari flap (ureteroneocystostomy) or even nephropexy. Proximal injuries can be managed using transureteroureterostomy, autotransplantation or ureteral replacement with bowel of appendix [14, 30, 43].
Bladder Trauma
The paediatric bladder is less protected than the adult bladder, and is therefore more susceptible to injuries than the adult bladder, especially when it is full, due to:
- · The pediatric bladder has a higher position in the abdomen and is exposed above the bony pelvis.
- · The abdominal wall provides less muscular protection.
- · There is less pelvic and abdominal fat surrounding the bladder to cushion it in trauma.
Classification
Blunt injuries to the bladder are categorized as:
contusions with damage to the bladder mucosa or muscle, without loss of bladder wall continuity or extravasation, or, ruptures, which are either intraperitoneal or extraperitoneal.
Incidence
Blunt trauma is the most common cause of significant bladder injury. In adults, bladder injury is often associated with pelvic fractures. This is less common in children because the paediatric bladder sits above the pelvic ring. Thus, only 57% of children with pelvic fractures also had a bladder injury compared to 89% of adults [30, 43].
Presentation
The characteristic signs of bladder injury are suprapubic pain and tenderness, an inability to urinate, and gross (microscopic) hematuria (95% of injuries). Patients with a pelvic fracture and gross hematuria present with a bladder rupture in up to 45% of cases [30].
Diagnosis
Bladder injuries are diagnosed by retrograde cystography [3, 7].
An appropriate retrograde cystogram for trauma requires that the bladder be filled to capacity, emptied and look for extravasated contrast. Films must be taken in the anteroposterior, lateral and both oblique alignments [14].
NB: If blood is noted at the urinary meatus, urethral injury must be ruled out with a retrograde urethrogram (RUG) before inserting a catheter for the cystogram.
Capacity of the bladder at various ages can be calculated by the following formulae:
· Bladder capacity in an infant in mL = 38 + [2,5 x age in months]
· Bladder capacity in the older child in mL = [age in years + 2] x 30
Management
Contusions usually present with varying degrees of haematuria and are treated with catheter drainage alone.
Intraperitoneal injuries
The accepted management is open surgical exploration and primary repair. Post-operative drainage with a suprapubic tube is mandatory. Recent data suggest that transurethral drainage may be as effective, with fewer complications, resulting in shorter periods of diversion. Usually, after about 7-10 days, a repeat cystogram is performed to ensure healing is taking place properly [3, 30, 43].
Extraperitoneal injuries
Non-operative management with catheter drainage for 7-10 days alone is the method of choice. However, if there are bone fragments within the bladder, these must be removed and the bladder must then be repaired and drained, according to the principles for treating intraperitoneal ruptures [30, 43].
Urethral Trauma
Except for the penile part of the urethra, the paediatric urethra is quite well protected. In addition, its shape and elasticity mean the urethra is seldom injured by trauma [3, 7, 8].
Urethral injuries in children are similar to those in adults, although injuriesto the prostate and bladder neck may be more common.
About 5% to 10% of boys with a fractured pelvis will also have an injury to the posterior urethra, usually at the proximal bulbar urethra. Motor vehicle accidents account for 90% of posterior urethral injuries, and the remaining 10% result from falls, crush injuries, or sporting injuries [43].
Boys are more likely than girls to get urethral injuries. That's because their urethras are much longer than girls' urethras and less protected by surrounding soft tissue. Therefore, female urethral injuries are rare and almost always related to pelvic fractures or cuts, tears, or direct trauma to the vaginal area.
Urethral injury should be suspected in any patient with a pelvic fracture or significant trauma to the perineum until confirmed otherwise by a diagnostic work-up [30].
Classification
Ureteral trauma is classified according to the AAST organ injury scaling system by the anatomic location of the injury and by the extent of mural damag (Table 8.6).
Table 8.6
|
Urethra Injury Scale of the AAST |
||
|
Grade |
Type of injury |
Description |
|
I |
Contusion |
Blood at urethral meatus; urethrography normal |
|
II |
Stretch injury |
Elongation of urethra without extravasation on urethrography |
|
III |
Partial disruption |
Extravasation of urethrographic contrast medium at injury site, with contrast visualized in the bladder |
|
IV |
Complete disruption |
Extravasation of urethrographic contrast medium at injury site without visualization in the bladder; <2cm of urethral separation |
|
V |
Complete disruption |
Complete transection with >2 cm urethral separation, or extension into the prostate or vagina |
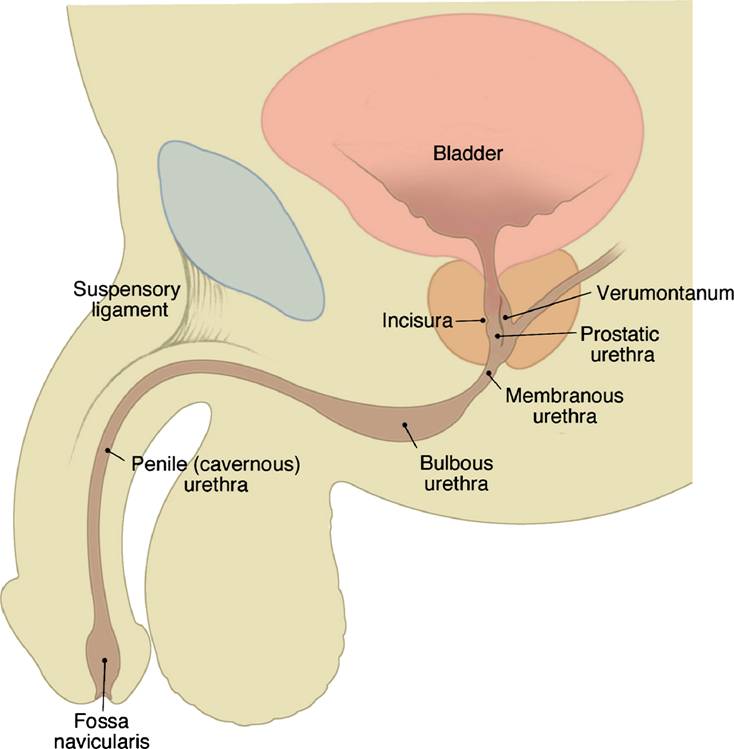
In the male, urethral injuries are also classified based upon whether they involve the posterior or the anterior urethra (Image 8.11).
|
|
Image 8.11 Normal male urethral anatomy.
Posterior urethra: •prostatic •membranous
Anterior urethra: •bulbar •penile
|
NB: The anterior urethra is composed of the penile and bulbar urethra to the level of the urogenital diaphragm. The posterior urethra is composed of the membranous and prostatic urethra. The posterior urethra extends from the bladder neck to the bulbous urethra.
Clinical Presentation
Children with urethral injuries are unable to void (or they have a pain during voiding) and are often seen with a distended bladder. Frequently, blood is noted at the external urinary meatus [3, 6, 8].
In posterior urethral injuries, rectal examination may reveal a pelvic hematoma or upward displacement of a distended bladder. Anterior urethral injuries are frequently associated with a perineal or scrotal swelling hematoma [2, 8].
Diagnosis
If there is suspicion of a urethral injury, blind passage of a transurethral urinary catheter should not be attempted because there is a risk of creating a false passage with the catheter and converting a partial disruption into a complete one [43].
Laboratory – urinalysis (gross haematuria).
Imaging - retrograde urethrogram is mandatory in suspected urethral trauma (Grade of recommendation: A) [3, 6, 8, 30].
If a catheter has already been placed by someone else and there is suspected urethral trauma, the catheter should be left in place and should not be removed. Instead, a small infant feeding tube can be placed into the distal urethra along the catheter to allow the injection of contrast material for a diagnostic scan [30].
If urethral integrity is demonstrated by retrograde urethrography, the catheter is the advanced and a cystogram is obtained to exclude concomitant bladder injury [43].
Treatment
Management of urethral injuries remains controversial due to the variety of injury patterns, associated injuries and treatment options. In addition, most urologists have little experience with these injuries and there is a lack of randomized prospective trials
Since many of these patients are unstable, the urologist's initial responsibility is to provide a method of draining and monitoring urine output.
NB: A transurethral catheter should only be inserted if there is a history of voiding after the traumatic event, and if a rectal and pelvic examination has not suggested a urethral rupture. If the catheter does not pass easily, an immediate retrograde urethrogram should be performed [30].
Female urethral injuries
These often occur together with bladder ruptures and can be repaired at the same time. A transvesical approach is best for proximal urethral injuries and a vaginal approach for distal injuries.
Male anterior urethral injuries
Blunt injuries
Grade I - II injury to the anterior urethra usually heals spontaneously without insertion of any indwelling urinary catheters, as long as the patient is able to void [14, 43].
Intermediate-grade anterior urethral injuries may be managed by an indwelling transurethral Foley catheter, whereas more complex injuries are best managed in the initial stages by placement of a suprapubic catheter [43]. A suprapubic tube may be placed percutaneously [3, 30].
A suprapubic cathether is inserted to drain the urinary bladder and antibiotics are administered. After 7 to 10 days a voiding cystourethrogram is carried out by instilling contrast through the suprapubic catheter. If the wound has healed, the catheter is clamped and the child is permitted to void. If no voiding problems are noted, the suprapubic catheter is removed.
If required, urethral reconstruction is generally delayed until the acute inflammatory process and hematoma have resolved.
Open injuries
Male urethral injuries: stab wounds, gunshot wounds and dog bites to the urethra often involve the penis and testes and require immediate exploration. During surgery, the urethral injury can be surgically evaluated and repaired [14, 43].
Primary repair should be aborted (in this case the situation is required the suprapubic urinary diversion) if primary anastomosis is not possible because of extensive disruption of the urethra, i.e. defects >1–1.5 cm long. Instead, the urethra should be marsupialized in preparation for a two-stage urethral repair, and a suprapubic urinary diversion should be considered. A delayed elective procedure should be carried out at a minimum of 3 months after injury.
Male posterior urethral injuries
Partial urethral rupture
Partial tears of the posterior urethra should be managed with a suprapubic or urethral catheter. Urethrography should be performed at 2-week intervals until healing has occurred [30, 43].
Complete urethral rupture
The initial management of complete posterior urethral injuries remains controversial, mainly regarding the long-term results with primary realignment compared to simple suprapubic drainage with later reconstruction [30, 43].
The main goals in the surgical repair of posterior urethral injuries are:
· providing a stricture-free urethra
· avoiding the complications of urinary incontinence and impotence
Complications
Complications of urethral injuries include stricture, incontinence and impotence.
Scrotal Trauma
Trauma to the scrotum occurs infrequently and severe injuries are unusual because of the size and mobility of the testes [14]. Trauma to the testes may result in a contusion to the testes or rarely, there can be a disruption of the covering of the testis and tunica albuginea [29].
Scrotal injuries may result from penetrating trauma or blunt trauma, or both.
Most injuries to the testicles are secondary to impact to the scrotum from rough play or sports [29].
Diagnosis
High-resolution ultrasonography is useful in the evaluation of these injuries, but it is operator dependant [3, 14, 43]. It may be best to explore the scrotum unless every part of the testis can be clearly identified on examination [14].
This technique is also useful in distinguishing less serious injuries, such as scrotal hematomas, hydroceles, and hematoceles, from surgical emergencies, such as testicular rupture and infarction [43].
Treatment
The treatment for scrotal trauma is dependent upon the underlying resulting injury.
If there is testicular or scrotal wall contusion without testicular injury, then treatment is supportive care with NSAIDs, scrotal support and limited physical activity [14, 29, 43].
If the testicle is ruptured or a large hematocele is observed, exploration is indicated to control bleeding, drain the hematocele and repair the torn tunica albuginea [3, 8, 14, 29].
Antibiotics are administered to avoid secondary infection.
Penetrating injuries usually require debridement and repair if the testis is involved [2, 3, 6]. An isolated scrotal wall hematoma is not amenable to drainage as the bleeding occurs between the layers of the scrotal skin and not in a cavity.
Penile Trauma
Penile injury resulting from blunt or penetrating trauma is rare in children [43].
Penile injuries are usually the result of the penis being caught in a zipper or of having the toilet seat drop on it.
Diagnosis
¤ The diagnosis of penile trauma is made on clinical examination.
¤ All penile injuries, other than very superficial injuries, should be evaluated with retrograde urethrography to exclude concomitant urethral injury [43].
Treatment
Gentle antibacterial cleansing three times a day is usually the only treatment needed to prevent secondary infection for superficial penile injuries [14]. For penile edema, treatment is supportive care with NSAIDs and limited physical activity.
Penetrating trauma requires exploration, evaluation of the urethra by urethroscopy and debridement with repair. The management of severe injuries of the penis requires an individualized surgical approach which may involve microvascular reconstruction and the use of skin flaps or skin grafts [3, 14].
Penile amputation injuries are rare and devastating injuries in children, but reimplantation and reconstructive techniques have been described with variable functional and cosmetic outcomes [43].
Labial Trauma
Blunt genital trauma in girls is fairly common. These injuries most commonly results from straddle injury and fall [3, 14, 43, 45]. Straddle injuries may cause large labial hematomas [14].
The most common types of injury in decreasing order of frequency are lacerations or contusions of the perineal body, vagina, labia, urethra, and rectum [43].
The presenting symptoms are usually the presence of blood in the underpants or on the perineum shortly after injury [2, 43]. Ecchymoses, abrasions, and hematomas are typical [45]. The external urinary meatus may be inflamed, but the female urethra is generally not injured [14].
Diagnosis
Anesthesia is often required to perform an adequate clinical examination because of the extreme difficulty of performing a thorough genitourinary examination in an awake, uncomfortable, anxious, and embarrassed child [14, 43].
Treatment
Management of female genital trauma is dictated by the type and extent of injury.
Most of these injuries are self-limited. Even a sizable vulvar hematoma will resolve on its own given time. Acutely after the injury, an ice pack may be useful to prevent tissue swelling, which causes the major morbidity in the situation [45].
Sitz baths and/or NSAIDs may successfully relax the area to permit voiding. Urethral catheterization is necessary if the child is unable to void [45].
Vulvar hematomas may cause urinary retention (in situations where the hematoma continues to grow despite conservative therapy) and may benefit from placement of a urinary catheter and evacuation of large hematomas [14, 43, 45].
Occasionally, a vulvar hematoma may be only the outward manifestation of a more serious deep injury such as a pelvic fracture
Necrotic contused tissue should be debrided. Lacerations are primarily repaired after hemostasis is achieved. Absorbable sutures are used to preclude the need for removal.
Vaginoscopy, urethroscopy, and proctoscopy may be necessary to evaluate the injury more thoroughly [43].
Sexual Abuse
Sexual abuse must always be kept in mind when evaluating children with perineal injuries [14, 45].