Inflammatory Surgical Diseases of Abdomen
Appendicitis
Appendicitis is an irritation, inflammation and infection of the appendix (a narrow, hollow tube that branches off the large intestine).
The appendix functions as a part of the immune system during the first few years of life. After this time period, the appendix stops functioning and other organs continue helping fight infection
|
Image 5.1/1 The positions of the appendix |
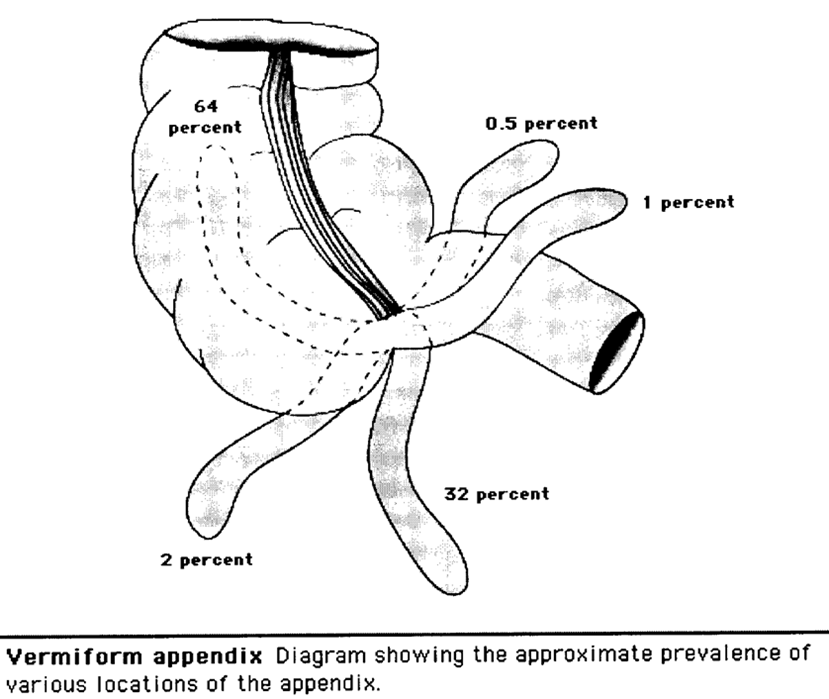
Image 5.1/2 The retroperitoneal position of the appendix.
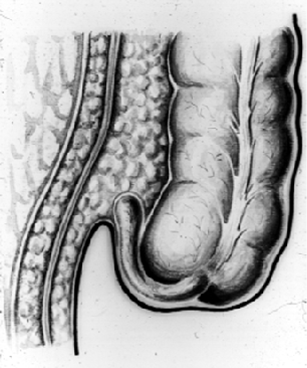
Image 5.1/3 Different localization of the cecum (therefore localization of abdominal pain could be different).
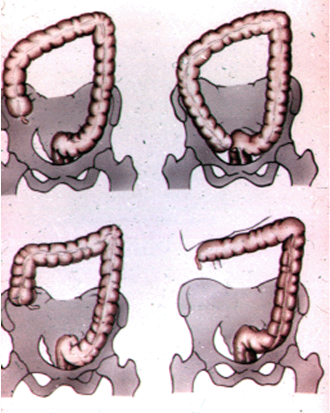
Image 5.1/4 Left-sided localization of the appendix (It can happens in case when the patient has a malformation - e.g. rotation of midgut does not complete).
Anatomy
The appendix develops from the cecum, which first appears during the 5th week as a ventral enlargement of the midgut.
The appendix has multiple possible positions in abdominal cavity (Image 5.1/1). Most common position of appendix is retrocaecal (64%) followed by pelvic (32%) (usually the appendix may lie across the psoas muscle or over the pelvic brim, resting on the pelvic fascia that overlies the obturator internus muscle). Other common positions are preileal and postileal. Retroileal position of appendix is the least common (0,5%) [2, 14, 21].
The size and shape of the appendix also vary. The appendix averages 11 cm in length but can range from 2 to 20 cm [45]. The diameter is typically less than or equal to 6 mm.
Epidemiology
Appendicitis is the most common cause of emergency surgery in childhood. Approximately 1% of all children younger than 15 years of age develop appendicitis with a peak incidence between 10 and 12 years of age [36, 43, 44].
The risk of developing appendicitis is lowest in infancy, perhaps because of the relatively wide base of the appendix at that stage of development. The risk of developing appendicitis that progresses to perforation is greater in children than in adults [2, 7].
In published series of appendicitis from children's hospitals, the incidence of perforation is 20% to 76% [14, 43]. This may be a consequence of the difficulty in making the diagnosis of acute appendicitis in the toddler or preschool-age child who cannot communicate as effectively as the older child.
Appendicitis affects males more often than females (a male-to-female ratio of 3:2). Acute appendicitis occurs more frequently during the summer months [6, 8].
Microbiology
Enteric bacteria are the most common organisms associated with appendicitis [14].
In the patients with perforation, Escherichia coli, Enterococcus, Bacteroides, and Pseudomonas are the species most frequently isolated from the abscess [43].
The possible role of viral infections in appendicitis has been implicated by the frequent prodrome of symptoms with which children with appendicitis may initially present [8, 14].
It is hypothesized that the viral illness may result in lymphoid hyperplasia or lymphadenopathy, which can obstruct the lumen. In addition, the viral illness may result in dehydration leading to a higher likelihood of inspissated stool or mucous leading to fecalith formation and obstruction [14].
Appendicitis has been described following Varicella infections [36, 43].
Parasitic infections with Enterobius or Ascaris have also been reported in association with appendicitis. These organisms may cause a local inflammation or may contribute to luminal obstruction leading to bacterial invasion and supurative appendicitis [16, 45].
Pathophysiology
Appendicitis is caused by obstruction of the appendiceal lumen that leads to vascular congestion, ischemic necrosis, and subsequent infection.
The most common cause of the obstruction is a fecalith or inspissated fecal matter [6, 21]. Fecaliths are identifiable in about 20% of children with appendicitis [14].
Other causes of appendiceal obstruction include:
- lymphoid follicle hyperplasia,
- carcinoid or other tumors,
- foreign bodies (i.e., pins, seeds, etc.), and
- rarely parasites.
1 - mucosa; 2 - lymphoid follicles; 3 - submucosa; 4 - muscular layer; 5 - serosa
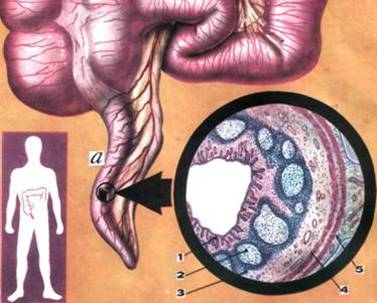
The obstructed appendix continues to secrete mucus causing the appendix to distend. Distension activates visceral nerve pain fibers that cause pain symptoms referred to the perium-bilical area (T-10 dermatome). As the intraluminal pressure increases, lymphatic drainage is impaired causing further edema and intramural pressure within the appendix. As the pressure continues to rise, venous outflow is compromised which leads to decreased arterial perfusion and ischemic necrosis [14, 36, 45].
Tissue infarction, gangrene with bacterial infection, and perforation follow if the condition remains untreated.
After perforation, localized abscess or diffuse peritonitis can occur. Diffuse peritonitis is common in young children and infants whose omentum is proportionately smaller and less able to contain an advancing suppurative process [43, 45].
Classification of appendicitis
|
|
|
Rare types of appendicitis include the following:
· Chronic appendicitis: Chronic appendicitis occurs with an incidence of 1% and is defined by the following:
* the patient has a history of RLQ pain of at least 3 weeks’ duration without an alternative diagnosis;
* after appendectomy, the patient experiences complete relief of symptoms;
* histopathologically, the symptoms were proven to be the result of chronic active inflammation of the appendiceal wall or fibrosis of the appendix.
· Recurrent appendicitis: The incidence of recurrent appendicitis is 10%. The diagnosis is accepted as such if the patient underwent similar occurrences of RLQ pain at different times that, after appendectomy, were histopathologically proven to be the result of an inflamed appendix.
There is some debate about the existence of recurrent or chronic appendicitis. It is difficult to reconcile the possibility of appendicitis resolving without some therapeutic intervention. Nonetheless, approximately one-fourth of patients with surgically proven acute appendicitis report a history of prior episodes of abdominal pain that is similar to the ones. Histopathology of resected appendices may show both chronic and acute inflammatory infiltrates and fibrosis, implicating prior episodes of acute appendicitis.
Like postcholecystectomy syndrome and irritable bowel syndrome, chronic appendicitis may be difficult to define and diagnose, but there are patients whose disease appears to fit the diagnosis.
· Spontaneously resolving appendicitis: This occurs if the cause of the symptoms is lymphoid hyperplasia or when a fecalith is expelled from the lumen.
Clinical Presentation
The diagnosis of acute appendicitis in childhood can sometimes be difficult. Definite diagnosis is made in only 50-70% of patients at the time of initial assessment. The rate of negative pediatric appendectomy is in the range 10-50% in various reports [12, 36, 43].
Symptoms of appendicitis may take 4-48 hours to develop.
The most common symptoms are listed in Table 5.1 [45].
Table 5.1.
|
Symptom |
Frequency (%) |
|
Anorexia |
95 |
|
Nausea/vomiting |
85 |
|
Fever |
60-80 |
|
Right lower quadrant pain |
70 |
|
Diarrhea |
10-30 |
History
The classic presentation for appendicitis involves the onset of vague epigastric or periumbilical pain followed by anorexia, nausea or vomiting and the migration of pain to the right lower quadrant (RLQ).
Owing in part, however, to the large variability in the location of the appendix, only 50-60% of patients will present in this classic history. For example, retrocecal appendicitis may present with vague and poorly localized pain while pelvic appendicitis can cause midline or left-sided abdominal pain.
The historical factors with the highest predictive value for appendicitis are RLQ pain (positive likelihood ratio (LR) of 7.31-8.46), migration of pain from the periumbilical region to the RLQ (positive LR of 3.18), and pain before vomiting (positive LR of 2.76).
Anorexia is the most common associated symptom with a sensitivity of 68% but lacks predictive value as it is commonly seen in other abdominal conditions. A history of similar pain does not exclude appendicitis but lowers the likelihood and should prompt consideration of alternative diagnoses.
It is important to recognize that symptoms commonly associated with other disease processes can also be seen with appendicitis. Constipation or diarrhea (especially in children) can occur and should be not used in isolation to exclude appendicitis. Similarly, irritative voiding symptoms (e.g., dysuria or frequency) can occur when an inflamed appendix is lying near the ureter or bladder.
Physical exam
Most specific finding: Rebound tenderness, pain on percussion, rigidity, and guarding (in the McBurney's point area).
NB: Location of the pain and tenderness is important.
! Abdominal rigidity (involuntary guarding) in RLQ is the sign with the highest predictive value for appendicitis (positive LR of 3.76).
|
|
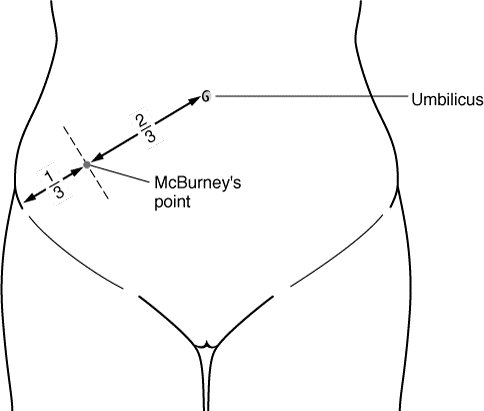
Image 5.1. The McBurney's point - is the point over the RLQ that is one-third of the distance from the anterior superior iliac spine to the umbilicus. This point roughly corresponds to the most common location of the base of the appendix where it is attached to the cecum. |
The following signs have been associated with appendicitis but have low predictive value (Table 5.2):
Table 5.2
Signs in Appendicitis
|
Sign |
Description |
Implication |
|
McBurney's sign |
Deep tenderness at McBurney’s point |
|
|
Psoas |
Pain with passive extension of right hip or with active flexion of right hip against resistance |
Retrocecal appendix |
|
Obturator |
Pain with passive internal rotation of right hip |
Pelvic appendix |
|
Rovsing |
Pain in RLQ with palpation of LLQ |
|
Fever is typically absent during the initial onset of pain but commonly develops within the first 24 hours. Overall, fever has poor predictive value for appendicitis.
NB: Infants and young children (age less than 5) tend not to be diagnosed until perforation has occurred. In addition to limited communication skills, young children pose a diagnostic challenge due the non-specific nature of their symptoms (i.e., abdominal pain, fever, and vomiting) as well as their high prevalence of atypical symptoms such as diarrhea, grunting, or a limp. Diarrhea was reported in 41% of cases of appendicitis in children less than 3 years old with gastroenteritis not surprisingly being the most common misdiagnosis.
Differentiation
Acute appendicitis can mimic just about any intra-abdominal process.
The differential of acute appendicitis is extensive and includes: gastroenteritis, Crohn’s disease, mesenteric adenitis (i.e., Campylobacter, viruses, Yersinia, etc.), pancreatitis, peptic ulcer disease, cholelithiasis, cholecystitis, Meckel’s diverticulitis, constipation, intussusception, and many other conditions.
Systemic disorders that are in the differential of acute abdominal pain and appendicitis include: porphyria, sickle cell crisis, Henoch-Schonlein purpura, hemolytic uremic syndrome, diabetic ketoacidosis, measles, Lupus erythematosus, and parasitic infections.
In females: ectopic pregnancy, ovarian torsion, ovarian cysts, and pelvic inflammatory disease must also be considered.
Urinary tract disease (i.e., renal stones, pyelonephritis, cystitis) can also mimic acute appendicitis.
Pneumonia, particularly of the right lower lobe, is a frequent nonabdominal source of lower abdominal pain in children that must be considered.
NB: In children less than 3 years old, gastroenteritis and ileocolic intussusception are the two most common conditions included in the differential diagnosis.
Acute appendicitis is associated with several other conditions. Patients with enterocolitis (Yersinia, Salmonella, Shigella, etc.) or parasitic infections (Entamoeba, Strongyloides, Enterobius, Schistosoma, Ascaris) can develop appendicitis secondary to both local or generalized lymphoid hyperplasia and obstruction of the appendiceal lumen. Viral infections with measles, chicken pox or cytomegalovirus (CMV) have been linked with appendicitis.
Children with cystic fibrosis have a higher incidence of acute appendicitis due to abnormal mucous that becomes inspissated and obstructs the lumen of the appendix. Hirschsprung’s disease should be considered in any neonate that presents with appendicitis.
Diagnosis
The patient’s history and clinical examination are the most important tools for the diagnosis of appendicitis [3, 7].
Laboratory investigations and plain radiographs are neither sensitive not specific in the diagnosis of appendicitis.
Laboratory Tests
There is not a single laboratory study specific for appendicitis.
· Leukocytosis (>10,000/mm3) is observed in 70-90% of patients with acute appendicitis. However, elevated levels can be noted with other conditions, and normal white blood cell (WBC) levels are often present with appendicitis. Neutrophilia is also observed (95%).
· It has been suggested that high levels of C-reactive protein (>0.8 mg/dL) in association with leukocytosis and neutrophilia are the most sensitive laboratory findings for appendicitis, with a sensitivity of approximately 97-100%. Therefore, the probability of acute appendicitis is low in the absence of these 3 laboratory findings.
· Urinalysis is important for determining the pregnancy status in childbearing females and ruling out urinary tract infection. However, there is a possibility of a microscopic pyuria or hematuria caused by the proximity of the appendix to the ureter and bladder in acute appendicitis. The presence of more than 20 WBC per high-power field in the urine is more suggestive of a urinary tract disorder.
Instrunental Tests
Radiologic Examinations
In general, plain radiographs are not useful in making the diagnosis of appendicitis. An opaque fecalith can be identified in the right lower quadrant in less than 5% of patients.
Radiographic studies should be reserved for the children whose symptoms are atypical or confusing.
A chest radiograph is also obtained to evaluate children with history “atypical” for appendicitis and suspected of having a pneumonia.
Ultrasonography
Sensitivity of test is 75-94%; specificity - 90% and an overall accuracy - 90% [14, 36, 43].
The main sonographic criterion for diagnosis is demonstration of a noncompressible appendix larger than 7 mm in diameter. Identification of an appendicolith or periappendiceal fluid is also helpful. As with other radiographic studies, the value of ultrasound may be to exclude other diagnoses, particularly in female patients.
The accuracy of ultrasound for appendicitis improves when the patient is able to indicate the area of maximal tenderness, but its negative predictive value is markedly diminished if the appendix is not visualized. Furthermore, false negatives are more likely in cases of perforated appendicitis (where the appendix is usually decompressed), and inconclusive studies more common in those with significant bowel gas and obesity.
Ultrasound offers the advantage of avoiding exposure to ionizing radiation and is thus the initial imaging study of choice in pediatrics and pregnant patients. It is also useful in women of childbearing age in whom gynecological causes of right lower abdominal pain are also being explored.
NB: In pediatric patients, American College of Emergency Physicians (ACEP) clinical policy recommends ultrasonography for confirmation, but not exclusion, of acute appendicitis; to definitively exclude acute appendicitis, the ACEP recommends CT.
Computed Tomography
CT has become the imaging study of choice for evaluating acute appendicitis in adults. In children CT may be helpful in selected cases but is rarely needed.
Magnetic Resonance Imaging
MRI has limited use in the diagnosis of appendicitis. Although MRI avoids ionizing radiation, it has several disadvantages including cost, limited availability on an emergent basis, and study length.
Laparoscopy
The use of the laparoscope is gaining popularity in the management of children with abdominal pain consistent with possible appendicitis. The rationale for its use is that identification of some other cause of pathology or a normal appendix would obviate the need for a negative appendectomy with its incumbent risks. There are specific patient populations that have a high incidence of negative laparotomy, such as postpubertal girls. Laparoscopy has been especially advocated in that group of patients [43].
Furthermore, some advocates of laparoscopy recommend that the appendix be removed via the laparoscope even if the appendix appears grossly normal [14, 43].
Treatment
For most patients, immediate surgical intervention is not considered mandatory. The patient with appendicitis is resuscitated with intravenous fluids, started on broad-spectrum IV antibiotics and kept NPO (NPO is a term used by hospitals which is short for the Latin words nil per os, which means nothing by mouth) [14, 36, 45].
Although spontaneous resolution can occur, appendectomy is still the treatment of choice for all patients suspected of having acute appendicitis [1, 8, 36].
All patients with appendicitis and generalized peritonitis require expedient resuscitation and urgent exploration.
Types of appendectomy:
v Open
- Antegrade (typical)
- Retrograde
v Laparoscopic
At open surgery, the abdomen is explored via a transverse or oblique right lower quadrant incision. The peritoneal cavity is entered and the appendix is delivered into the wound if possible. The appendix is assessed for signs of inflammation, gangrene and/or perforation.
The appendix can usually be removed by the standard technique of stump ligation after transection, with or without inversion of the stump into the base of the cecum. If pus is present, the abdomen should be irrigated with saline. Drains are not necessary. The abdominal wall is closed in layers. The skin is usually closed by subcuticular absorbable sutures, even in the case of perforation.
In rare cases, when the cecal wall is involved in a gangrenous, inflammatory process, limited ileocecal resection with primary anastamosis may be necessary.
If a normal appendix is found at laparotomy (5-15% of cases), the abdomen is systematically inspected for evidence of inflammatory bowel disease, a Meckel's diverticulum, mesenteric adenitis, peptic ulcer disease and other pathology. In females, the ovaries should be identiied and inspected. If Crohn's disease is encountered, the appendix should be removed unless the disease process grossly involves the base of the appendix.
Postoperative care includes continued broad-spectrum antibiotic therapy.
Antibiotic therapy must provide activity against the common pathogens associated with appendicitis: E. coli, Bacteroides, Enterococcus and Klebsiella.
A switch to oral antibiotics is sometimes made when the patient is afebrile and tolerating a diet. For perforated or gangrenous antibiotics, a 5-day course is the usual recommended therapy but many surgeons stop postoperative antibiotics when the recovering patient is afebrile with a normal WBC [14, 16, 36, 43].
Complications of acute appendicitis:
v Preoperative complications:
- Peritonitis
- Periappendicular mass (or abscess)
- Cellulitis of retroperitoneal space
- Sepsis
- Pylephlebitis
v Postoperative complications:
Complications of the surgical wound:
- Tissue damage at the operation site
- Haematoma
- Seroma
- Wound infection
- Haemorrhage
- Spitting sutures (the suture is pushed out to the skin surface by the body rejecting the suture)
Complications of the abdomen cavity:
- Peritonitis
- Periappendicular mass (or abscess)
- Abdominal abscess (ileocecal, Douglas space, subdiaphragmatic)
- Bowel obstruction
- Intestinal fistula
- Intraabdominal bleeding
Complications unrelated to the operating area:
- On the side of respiratory system (pneumonia, bronchitis, acute upper respiratory tract infections)
- Other complications (myocarditis, pericarditis, pyelonephritis)
NB:
· Be aware that normal temperature or WBC does not rule out appendicitis
· There is no single sign, symptom, or lab that completely rules out appendicitis
· Urinanalysis with pyuria or hematuria can be appendicitis due to lie of inflamed appendix
· The localization of pain can be atypical due to the anatomic position of appendix as with, for example, a retrocecal or pelvic appendix
· Ultrasound should be used as the first imaging study in children and pregnant females
· Extremes of age (infants and young children (age less than 5) and elderly) have atypical presentations necessitating a high index of suspicion
· In females presenting with RLQ pain and tenderness, make sure gynecologic diseases have been appropriately considered including ectopic pregnancy, ovarian torsion, or tuboovarian abscess.
· Patients evaluated for appendicitis who are discharged after a negative imaging study should be cautioned that appendicitis is still a possibility and advised to be rechecked in 12-24 hours if still having pain (false negatives are possible)
Special considerations
Perforated appendicitis
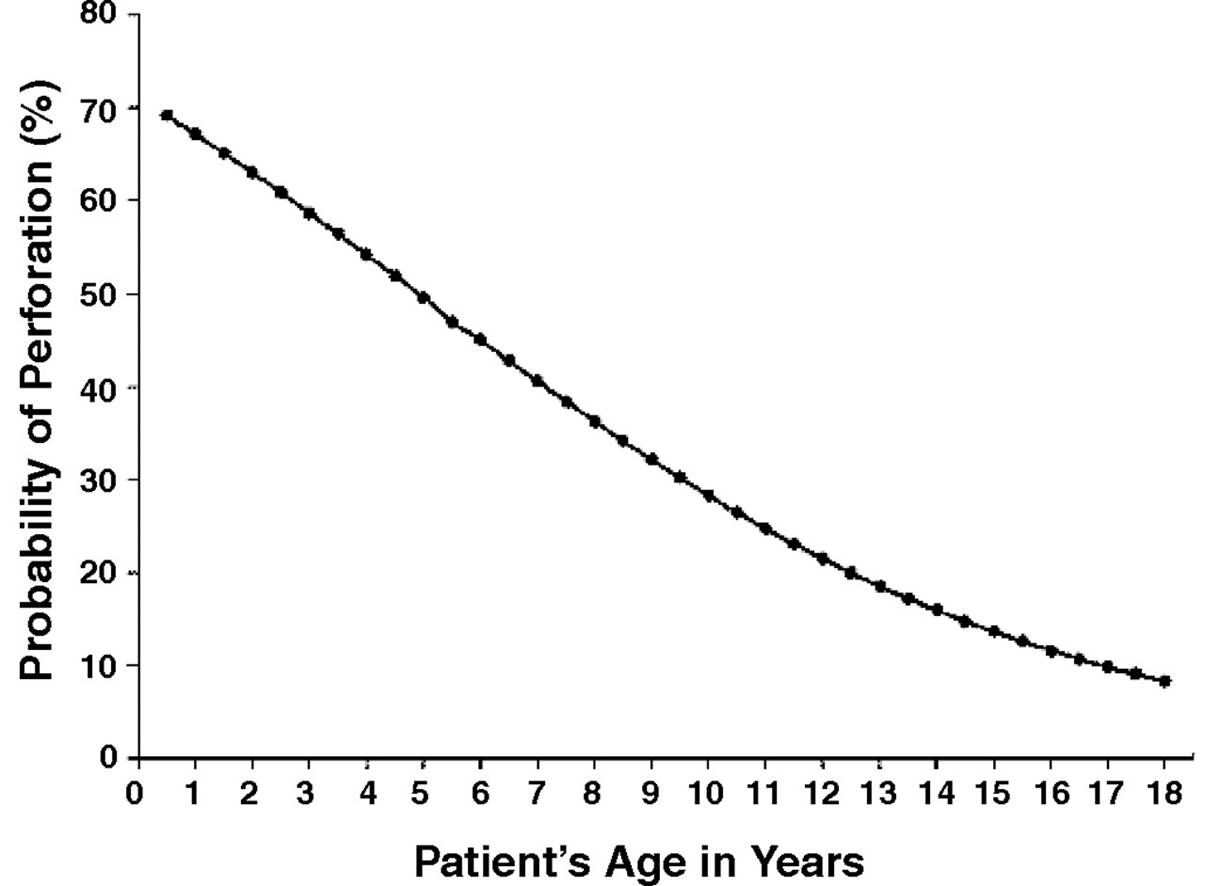
The appendicitis leading to perforation is more common in children than in adults (Fig. 5.1). The large diameter relative to the cecum and thin wall of the appendix in childhood may be contributing factors that predispose children to a more rapid progression of the disease [12].
|
Figure 5.1 Theoretic curve showing inverse relationship between predicted perforation of appendix and patient's age [12]. |
The risk of perforation within 24 hours of the onset of symptoms is less than 30%. If symptoms have been present for more than 48 hours, the probability of perforation is greater than 70% [14].
The symptoms of perforated appendicitis evolve from a transient decrease in the abdominal pain secondary to the release of pressure in the appendix, to a severe generalized abdominal pain, fever often higher than 38°C, worsening anorexia with nausea and vomiting, dehydration, and diarrhea (which can be misleading by suggesting the diagnosis of gastroenteritis) [3, 8, 14].
Appendiceal (periappendicular) mass
Between 2–6% of cases of acute appendicitis are complicated by development of a periappendicular mass, ranging from a phlegmon to an abscess [45]. The periappendicular mass is a limitation of inflammation in abdominal cavity by peritoneum and omentum.
NB: Appendicitis with a palpable mass is usually a consequence of perforation.
An intra-abdominal abscess can occur during two phases of appendicitis (Table 5.3):
- Either during initial patient presentation as an aspect of ruptured appendicitis
- Postoperatively as a complication of advanced appendicitis
Table 5.3
|
Appendiceal Mass |
|
|
- as an aspect of ruptured appendicitis before appendectomy |
- as a complication of advanced appendicitis after appendectomy
|
|
Patients usually present with abdominal discomfort, anorexia, change in bowel habits, fever, and other nonspecific symptoms. Typically, there is a long duration of symptoms and patients often present after 5–7 days of symptoms. * The patients more than 10-14 years of age are affected usually. |
Typically, these patients do not improve following appendectomy and develop recurrent fevers, abdominal pain, anorexia, and elevated WBC. These symptoms should lead to an evaluation for the presence of a postoperative intra-abdominal abscess.
* The incidence of postappendectomy intra-abdominal abscess is between 5–20% |
Diagnosis
¤ On physical examination an abdominal mass may be present but abdominal wall guarding may preclude palpation.
Typically, fever and WBC are higher than in uncomplicated appendicitis.
¤ Abdominal CT is useful to determine whether an appendiceal abscess is present, to differentiate the abscess from a phlegmon, and to determine whether the abscess is amenable to percutaneous drainage [36, 43].
¤ High-resolution ultrasonography with graded compression also has very good sensitivity and specificity [36].
Treatment
The management of patients who present with abdominal masses in the setting of appendicitis is controversial.
The acute inflammation of the cecum and surrounding bowel can result in long, difficult operations with more blood loss, increased risk of bowel injury, and inability to safely perform a complete appendectomy.
If the mass is determined to be a phlegmon, then intravenous antibiotics and volume resuscitation without immediate laparotomy is a safe and effective treatment [43, 45].
The patient shows clinical improvement with lower temperature spikes, decreasing leukocytosis, and decreasing abdominal tenderness. The interval appendectomy can be performed 6 to 8 weeks later [14, 36, 43, 45].
If clinical improvement does not occur after 12 to 24 hours of this nonoperative management, then laparotomy is indicated [14, 45].
If a large abscess (about 2 cm in diameter) is identified, drainage by percutaneous, transvaginal, or transrectal routes is an effective treatment after resuscitation and intravenous antibiotics. The abscess drainage is usually performed by an interventional radiologist either percutaneously or transrectally using ultrasound or CT guidance [45].
NB: Patients with appendiceal abscess who present with signs of diffuse peritonitis, multiple abscesses or bowel obstruction, and appear toxic, typically are not candidates for percutaneous drainage and require early surgical intervention.
Small abscess (less than 2 cm in diameter) may be managed with antibiotics alone. As long as clinical improvement occurs, then antibiotic therapy is continued until the patient is afebrile, the WBC count is normal, and the abdominal tenderness is resolved. This postdrainage therapy can be completed as an outpatient. Interval appendectomy 6 to 8 weeks later is usually remarkably uncomplicated [14, 36, 45].
Acute appendicitis in Infants
Appendicitis in neonates is very uncommon and has a mortality rate of 50% to 80% [12]. If identified in the neonatal period, appendicitis should be treated with emergent operation and rectal biopsies because Hirschsprung's disease is frequently the underlying cause [14].
Appendicitis in children between 1 month and 1 year of age is unusual [16, 36]. These children are likely to present with perforated appendicitis and to manifest the complications of appendicitis, such as intestinal obstruction and systemic sepsis.
It is indeed rare and fortuitous to make the diagnosis of acute appendicitis before perforation in this age group. This is a serious illness in infants and has a mortality rate as high as 10% [12].
Postpubertal Females
An accurate diagnosis of the source of abdominal pain in postpubertal girls is often difficult. In addition to appendicitis, ovarian pathology such as an ovarian cyst ruptured (or intact), ovarian torsion, pelvic inflammatory disease, and pain with ovulation, or menstruation, can be common causes of severe lower abdominal pain.
In most series, the rate of negative appendectomies (i.e., appendices lacking histologic evidence of inflammation) is reported to be as high as 40% [14].
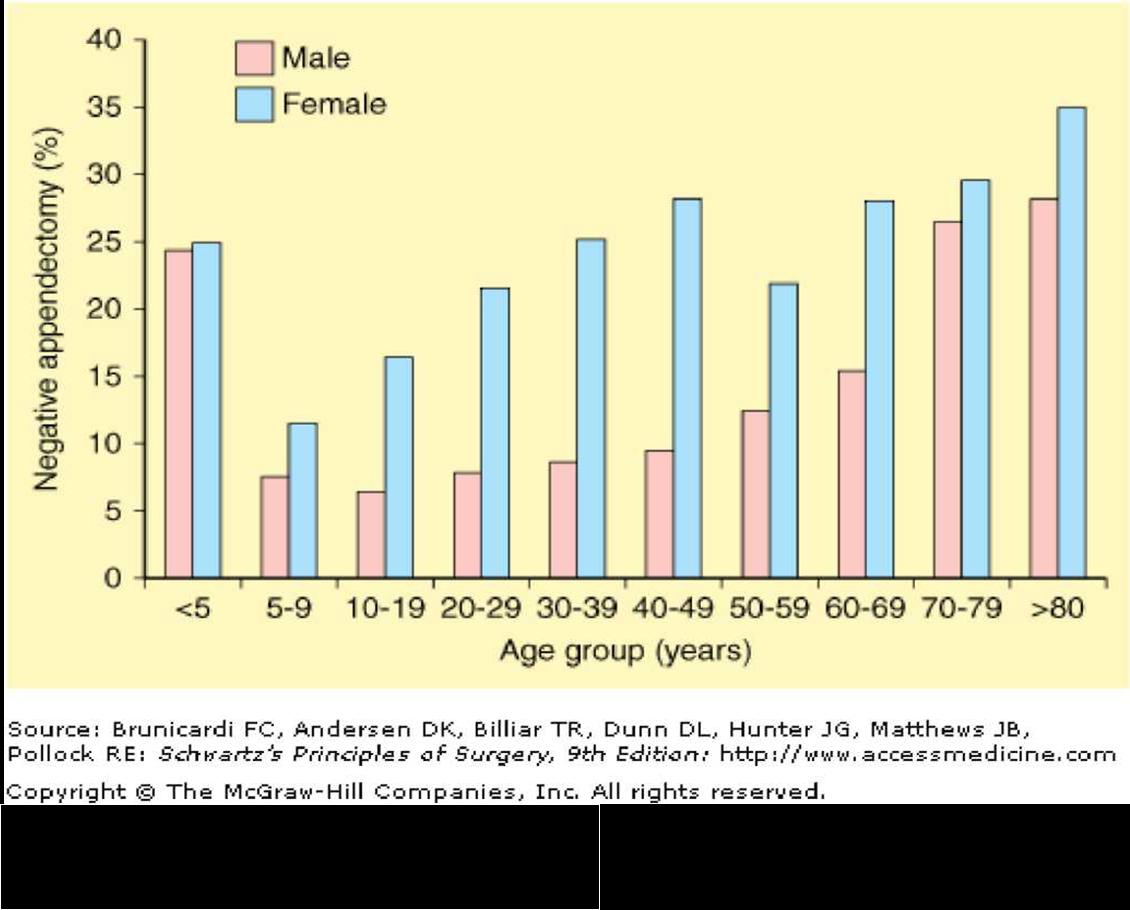
Ultrasound examinations may decrease the negative appendectomy rates because ultrasound can be valuable to assess both the ovaries and the appendix.
Diagnostic laparoscopy has been used as an effective way to define the cause of lower abdominal pain in this age group. There is some debate because many surgeons reason that the appendix should be removed during the laparoscopic procedure, regardless of its macroscopic appearance [14, 43, 45].
Gallbladder Disease in Childhood
Gallbladder disease is uncommon in infants and children and is generally classified as acquired or congenital.
For those who fall into the acquired category, 30% have neonatal cholestasis syndromes and 40% have calculous disease. Congenital anomalies are identifiable in only 14% of afflicted children [14].
The overall prevalence of cholelithiasis in neonates and young children is approximately 0,15-0,22%. This percentage increases in older children and teenagers [43].
The genders are affected equally until adolescence, when cholelithiasis is more common in females [2, 3, 8].
Children with hemoglobinopathies or hemolytic diseases are at great risk for cholelithiasis. Gallstones are identifiable in 12-40% of teenage children with sickle cell disease. Biliary sludge (without stones) is detectable in another 10-16%. Although biliary sludge progresses to cholelithiasis in 66-100% of children with sickle cell disease, resolution may occur in up to 20%. Approximately 14% of children with sickle cell disease undergo cholecystectomy during childhood [14].
Etiology
Lithogenesis: Gallstones are usually classified into one of three types:
· cholesterol stones
· pigment stones
· mixed-type
Cholesterol stones usually result from cholesterol saturation of bile and stasis and are found in children and adolescents without hemolytic disease. These stones are radiolucent and form within the gallbladder, sometimes migrating into the ductal system. Obesity, Western diet, pregnancy, and advancing age are risk factors for cholesterol stones [14, 36].
Pigmented stones are radiopaque and associated with supersaturation of bile with calcium bilirubinate, usually due to hemolytic disease. Brown stones are associated with bacterial cholangitis and can form within the ductal system [43].
Cholecystitis is inflammation of the gallbladder wall, often due to irritation by retained stones or sludge within the gallbladder.
Acalculous Cholecystitis
Acute or chronic acalculous cholecystitis occurs in children without gallstones.
Acute acalculous cholecystitis is not uncommon in children and is often associated with serious chronic illness. Although the etiology is poorly understood, risk factors include hyperalimentation, mechanical-assisted ventilation, ileus, and prolonged fasting [14].
Several factors may predispose children to develop acalculous cholecystitis including:
· dehydration
· adynamic ileus
· gallbladder stasis
· total parenteral nutrition
· hemolysis
· massive transfusions
These conditions are frequently encountered in children with severe critical illness (i.e., multisystem trauma, burns, pneumonia, sepsis, severe infection) [36, 43].
Biliary dyskinesia, or chronic acalculous cholecystitis, is a distinct clinical entity that is characterized by decreased gallbladder contractility and signs of chronic cholecystitis on histologic study after cholecystectomy [43].
This condition is now more commonly seen in adolescents with chronic or recurrent right upper quadrant pain.
Cholelithiasis in infancy is often related to hyperalimentation and is associated with a variety of risk factors, including congenital biliary tract abnormalities, parental nutrition, hemolytic disease, and prolonged fasting.
Obstruction of the common bile duct may occur due to gallbladder sludge and stasis.
Several factors associated with gallbladder disease in children are listed in Table 5.4 [43].
Table 5.4
|
Factors associated with cholelithiasis or cholecystitis |
|
|
Drugs/Treatments |
Association |
|
Exchange transfusion |
Stones |
|
Furosemide |
Stones, sludge |
|
Phototherapy |
Stones, sludge |
|
Morphine |
Stasis |
|
Ceftriaxone |
Pseudolithiasis |
|
Chemotherapy for Wilms' tumor, neuroblastoma, Hodgkin's disease, non-Hodgkin's lymphoma |
Stones |
|
Diseases/Conditions |
Association |
|
Obesity |
Stones |
|
Sickle cell disease |
Stones, sludge |
|
Polycythemia |
Stones, sludge |
|
Hereditary spherocytosis |
Stones, sludge |
|
Kawasaki's disease |
Hydrops |
|
Byler's disease (progressive familial intrahepatic cholestasis) |
Hydrops |
|
Hepatitis A |
Edema, wall thickening |
|
Epstein-Barr virus |
Hydrops, sludge |
Polypoid lesions of the gallbladder are rare in childhood. They may occur as a manifestation of metachromatic leukodystrophy, Peutz-Jeghers syndrome, or pancreaticobiliary malunion. Other, idiopathic polyps have a variable histology (adenoma, gastric heterotopia, cholesterol polyp, and epithelial hyperplasia). Cholecystectomy is recommended for idiopathic polyps if there are biliary symptoms or if the polyp is greater than or equal to 1 cm in size.
Clinical Presentation
The typical presentation of any gallbladder malady involves right upper quadrant abdominal pain, nausea and emesis [2, 3].
If infection is present, fever, leukocytosis, or Murphy's sign (an inspiratory pause due to patient discomfort when the examiner holds mild pressure in the right upper quadrant) may be present. Vomiting occurs in about one third of patients [14].
In younger children, the pain is often more generalized. Charcot's triad (fever, right upper quadrant pain, jaundice) suggests ascending cholangitis.
If obstruction to bile flow occurs, jaundice and acholic stools are seen. It is critically important to identify the etiology of the jaundice in order to provide proper treatment. If stones are present, surgery will correct the problem. However, more serious causes of jaundice (i.e., biliary atresia, choledochal cyst) must be excluded.
NB: Chronic cholecystitis and asymptomatic cholelithiasis are more common than acute cholecystitis in the pediatric population [36, 43]. Chronic cholecystitis presents with recurrent episodes of abdominal pain.
Acute acalculous cholecystitis is often diagnosed in association with another systemic disease state and is rarely seen. Patients are typically in critical condition with sepsis, massive burn, prior surgery or other widespread illness.
Neonates and younger infants frequently have an associated clinical condition (i.e., prolonged total parenteral nutrition (TPN), prematurity, cystic fibrosis (CF), prolonged fasting) that may contribute to cholestasis and jaundice.
In neonates, acalculous cholecystitis with a gangrenous gallbladder can mimic necrotizing enterocolitis. If initial medical therapy is unsuccessful, this diagnosis should be considered and exploration may be necessary.
Diagnosis
In addition to history and physical examination, laboratory evaluation of the patient's leukocyte count, electrolytes, serum glucose, liver function tests (AST, ALT, alkaline phosphatase, albumin) and amylase help formulate the differential diagnosis for any child with abdominal pain and emesis.
NB: Laboratory tests usually reveal a mild leukocytosis (12,000-15,000/mm3) and, if there is common bile duct obstruction from stones or edema, elevated conjugated bilirubin.
Transabdominal ultrasonography is both sensitive and specific to identify gallbladder disease [1, 3, 4].
Depending on the size of the child, endoscopic retrograde cholangiopancreatography (ERCP) or percutaneous transhepatic cholangiography (PTCA) are used to study the biliary system [14].
If there is no evidence of cholelithiasis or biliary dilation, nuclear studies are infrequently used to assess the function of the biliary tract. Cholescintigraphy is a nuclear medicine scan in which a technetium-99 labeled isotope is injected intravenously for concentration and excretion in the bile. Visualization of the gallbladder usually rules out cholecystitis; excretion into the duodenum rules out complete common bile duct obstruction. Cholescintigraphy may yield false positive results [14, 43].
Treatment
Identification of cholelithiasis should lead to prophylactic, elective cholecystectomy especially if the child has abdominal complaints that suggest cholecystitis or abdominal crises that can be confused with recurrent bouts of gallbladder inflammation [3, 16, 36, 43].
NB: Removal of the gallbladder is indicated for patients with symptomatic gallstone disease.
The management of patients with acute cholecystitis begins with stabilization of the patient and preparation for the operating room if the patient is a suitable candidate. Fluid resuscitation and a broad-spectrum antibiotic are administered.
Generally, most surgeons typically schedule the patient with acute cholecystitis for laparoscopic cholecystectomy within 1 day to 6 weeks of presentation [43].
NB: The laparoscopic approach has become the standard of care. The technique for laparoscopic cholecystectomy in pediatric patients is very similar to that described for adult patients.
Acute Pancreatitis
Pancreatitis is uncommon during childhood; however, it must be considered in every child with unexplained acute or recurrent abdominal pain [43].
Acute pancreatitis is defined as a single or recurrent episodes of abdominal pain associated with elevated serum pancreatic enzyme levels [3, 7, 8].
The morphologic correlate is acute focal or diffuse swelling and inflammation of the pancreas.
A continuum exists between acute and chronic pancreatitis; as a result, recurrent episodes of acute pancreatitis may evolve into the typical clinical and morphologic features of chronic pancreatitis.
Etiology
The vast majority of cases of pancreatitis in children are caused by blunt abdominal injury. In the pediatric population, nearly 40% of cases of traumatic pancreatitis are attributable to bicycle-related injury [7, 8, 14].
After trauma, the most common causes of pancreatitis in children are drug therapy (corticosteroids, azathioprine, thiazides, furosemide, tetracyclines and valproic acid), viral infection (Epstein-Barr, Coxsackie, enterovirus and mumps) and occasionally bacterial infection. Cystic fibrosis, biliary disease, vasculitic diseases (systemic lupus, Henoch-Schonlein purpura) and Type I and V hyperlipidemias are also associated with acute pancreatitis in the pediatric population (Table 5.5).
Table 5.5
Causes of Acute Pancreatitis in Children (by Jay L. Grosfeld, 2006).
|
Systemic infection
|
|
|
Trauma
|
|
|
Anomalies of the pancreaticobiliary duct system |
|
|
Drugs
|
|
|
Metabolic abnormalities |
|
|
Liver transplantation |
|
|
Miscellaneous |
|
|
Idiopathic |
|
In contrast to adults, cholelithiasis and alcohol are not major etiological factors for pancreatitis in children, with the exception of those with hemolytic diseases who develop gallstones.
Clinical Presentation
Children present with vague, mostly epigastric, abdominal pain which is exacerbated by eating.
The classic symptom of pain radiating to the back is rarely observed in the pediatric population [14].
Nausea and vomiting may be present. Fever may be seen as well. Rarely, patients may present with symptoms of small bowel obstruction. Some young women may develop salpingitis secondary to pancreatitis.
Diagnosis
The diagnosis of acute pancreatitis is based on the clinical history, physical examination, results of laboratory tests, and the findings of diagnostic imaging investigations.
¤ Serum amylase, trypsinogen and lipase levels are useful to establish the diagnosis of acute pancreatitis.
Because amylase production occurs from other nonpancreatic sources (i.e., salivary gland), elevated serum amylase is relatively nonspecific. Calculation of the amylase clearance may be helpful and is normally less than 5% [14].
Trypsinogen and lipase are produced almost exclusively by the pancreas; elevated serum levels are more specific for pancreatitis [14, 36, 43].
NB: Amylase clearance or Amylase/creatinine clearance ratio - this test calculates the ratio of amylase to creatinine that is filtered by the kidneys. It is used to evaluate pancreatic disease and to screen for a condition called macroamylasemia.
¤ CT is the best radiographic study to image the pancreas in cases of severe or complicated pancreatitis [36,43]. Abdominal CT is often obtained as part of the trauma evaluation.
¤ Ultrasound is sometimes useful, but often only provides limited visualization of the pancreas due to its retroperitoneal location and interposed bowel gas which further limits the study [14, 36].
¤ Endoscopic retrograde cholangiopancreatography (ERCP) is an invasive test that can accurately delineate pancreatic ductal anatomy. ERCP by itself may cause pancreatitis in 5-10% of cases and is generally avoided during the early phases of acute pancreatitis [14].
Treatment
Because numerous disease entities can cause pancreatitis, patient management must be highly individualized [23, 36, 43].
Conservative medical management is the mainstay of treatment for pancreatitis [3, 6, 8, 45].
Treatment of acute pancreatitis has two primary goals: (1) to minimize any causative factors and (2) to provide meticulous supportive care, including liberal use of analgesics, administration of parenteral fluids, maintenance of nutrition, prevention of infection, and inhibition of endocrine and exocrine activity [14].
Parenteral analgesia with narcotics or nonsteroidal anti-inflammatory agents is generally required, even in mild cases of pancreatitis, because the pain can be extreme. Meperidine is preferred because it does not cause sphincter of Oddi contraction as morphine does [16, 23, 43].
Nasogastric suction is usually initiated to reduce vomiting and abdominal distention; however, unless the patient is vomiting, the value of nasogastric decompression is questionable. Aspiration of gastric acid also may reduce pancreatic exocrine secretion by limiting the release of secretin [14, 23].
Oral feeding must be withheld to reduce pancreatic stimulation. Total parenteral nutrition should be initiated early to avoid malnutrition [3, 8, 45].
Enteral feeding distal to the ligament of Treitz via jejunal feeding tube is the preferred method of providing nutrition in refractory cases [14, 23, 43].
In addition, early placement of a central venous catheter in patients with severe disease will provide access for aggressive intravascular volume support and nutrition.
Although the advantages of prophylactic antibiotics have not been proved, patients with necrotizing pancreatitis may benefit.
Other treatment strategies involving somatostatin, glucagon, anticholinergics, histamine blockers, and protease inhibitors have been recommended, but to date they have not shown conclusive benefit [14, 43].
NB: The majorities of cases of pancreatitis are self-limited and resolve spontaneously with supportive therapy.
In severe cases (i.e., necrotizing pancreatitis, infected pancreatic necrosis), surgical intervention may be necessary for irrigation and/or debridement of the pancreas.
In the management of severe acute pancreatitis, a major decision is whether and when surgery for pancreatic necrosis or infection is necessary. Infection of necrotic pancreatic tissue is the major risk factor for mortality in severe acute pancreatitis. The morality rate in this scenario approaches 15% [14, 43].
Early surgical intervention may be necessary when the gland is totally transacted after trauma. This is most likely to take place near the midline, where the pancreas is crushed against the vertebral body. In such situations, distal pancreatectomy and oversewing the stump are the choice of treatment.
Prognosis
The prognosis in children is generally good, with the exception of pancreatitis occurring with multiorgan failure [2, 45].
Necrotizing Enterocolitis
Necrotizing enterocolitis (NEC) is the most common diagnosis that requires emergent operation in the neonate.
NEC is a medical condition primarily seen in premature infants, where portions of the bowel undergo necrosis.
The disease involves the culmination of three factors:
· premature
· immature immune system
· gastrointestinal tract colonized with pathologic bacteria
When these three factors are present in the setting of feeding an immature intestinal tract, an unknown trigger or series of events occur that together create the NEC [14].
Incidence
The incidence of NEC is difficult to ascertain. Reports generally indicate an incidence of 1-3 cases per 1000 live births. The incidence in infants weighing less than 1500 g is 10%. The risk of mortality is inversely proportional to birth weight [14, 36, 43].
NB: NEC typically occurs in the second to third week of life in the infant who is premature and has been formula fed.
Etiology
No specific etiology of NEC has been identified; multiple factors are believed to contribute to its development. The principal factors identified are prematurity and enteral feedings [3, 8, 16, 36, 45].
Prematurity is the major predisposing factor in the development of NEC. Premature newborns have immature gastrointestinal barrier defense mechanisms, limited by inadequate production of mucus, complement, immunoglobulins (i.e., IgA, IgM) and poor phagocyte function [14].
Exposure to antibiotics, pathogenic bacteria and formula feeds create a luminal environment suitable for bacterial overgrowth. If intestinal ischemia occurs, bacteria can translocate and NEC may develop. Additional factors that may contribute to the development of NEC are listed below:
Factors contributing to development of NEC:
- Umbilical catheters
- Hypotension
- Enteral feeds
- Pneumonia
- Maternal cocaine use
- Hyperosmolar formula feedings
- Vasoconstrictive medical therapy (indomethacin)
- Patent ductus arteriosus
NEC also occurs as an infrequent postoperative complication, most commonly following gastroschisis or myelomeningocele repairs [43].
Classification
The Bell system is the staging system most commonly used to describe NEC (Table 5.5). However, clinical distinction between each stage is often difficult.
Table 5.5
|
Stage |
Clinical Findings |
Radiographic Findings |
Treatment |
Survival |
|
I: Suspected NEC |
Emesis, mild distention, intolerance to feeds |
ileus pattern |
Medical evaluation, treat for NEC, sepsis evaluation |
100% |
|
II: Definite NEC |
Bilious emesis or gastric drain output, marked abdominal distention, occult or gross GI hemorrhage |
ileus, pneumatosis intestinalis, portal vein gas |
Aggressive medical resuscitation and therapy for NEC |
96% |
|
III: Advanced NEC |
Bilious gastric output, abdominal distention, occult or gross GI hemorrhage, abdominal wall erythema, deterioration of vital signs, septic shock |
ileus, pneumatosis intestinalis, portal vein gas, pneumoperitoneum, ascites |
Surgical |
50% |
Infectious organisms
Infectious organisms play a key role in the development of NEC. Whether bacterial infection has a primary inciting role in NEC or whether an initial intestinal mucosal injury allows secondary bacterial invasion is unclear [14, 43].
Positive blood cultures are found in 30% of patients; the most commonly identified organisms are Escherichia coli and Klebsiella pneumoniae [14, 45].
Proteus mirabilis, Staphylococcus aureus, Staphylococcus epidermidis, Enterococcus species, Clostridium perfringens, and Pseudomonas aeruginosa have also been identified.
E coli, Klebsiella species, Enterobacter cloacae, P aeruginosa, Salmonella species, S epidermidis, C perfringens, Clostridium difficile, and Clostridium butyricum commonly grow in stool cultures.
Klebsiella species, E coli, S epidermidis, and yeast are most commonly identified on peritoneal cultures. Fungal infection is believed to be an opportunistic infection in the presence of an altered host intestinal defense system.
Pathology
NEC can involve any segment of intestine, but it most commonly affects the ileocecal segment (45%) supplied by the most distal branches of the superior mesenteric artery [2, 3, 6].
Isolated small intestinal involvement is noted in up to 50% of cases and it is limited to the colon in 25% of cases, the splenic flexure being the most common site of colonic involvement. Pan-necrosis (involvement of more than 75% of the bowel) occurs in 14-30% of cases and almost all babies afflicted with this fulminant form of NEC die [14, 43].
The affected bowel is distended and its wall is thinned with hemorrhagic or graying areas. Subserosal or intravascular gas is observed in 50% of cases. Areas of bowel that are not perfused appear pale.
Histologically, coagulation necrosis of the mucosa is the predominant feature; however, full or partial thickness involvement of the subserosa and muscular layers is also common. Viable areas of bowel demonstrate features of acute and chronic inflammation. Granulation tissue, fibrosis and epithelial regeneration are signs of an extended duration of injury and recovery [14].
Clinical Presentation
NB: Although clinical presentation can be quite variable, early NEC presents with feeding intolerance.
A progressive ileus may also be present, resulting in abdominal distension, tachypnea, lethargy and gastric distension. Bilious vomiting may be noted in 75% of affected infants. Gross or occult blood is identified in the stool of 25-55% of patients and diarrhea occurs in up to 20% of patients [14].
Abdominal examination is notable for distension, diminished bowel sounds and tenderness. Initially the abdomen is soft but it often becomes firm and increasingly tender with erythema (5%), discoloration, abdominal wall edema and crepitance developing. A fixed abdominal mass may be appreciated in some cases. Tympany is elicited if free intraperitoneal air is present.
As the disease progresses, clinical indicators of shock and sepsis, such as temperature instability, increased lethargy, apnea, bradycardia and oliguria, become evident. Peripheral perfusion is diminished. Increasing oxygen requirement and need for intubation and mechanical ventilation are also signs of disease progression.
Laboratory testing reveals leukocytosis (predominantly neutrophils or bands) or leukopenia and thrombocytopenia, which occurs in response to Gram-negative septicaemia [3, 8, 14, 36].
In 60-90% of cases metabolic acidosis, as a result of intravascular volume depletion and hypoperfusion, may develop. Prothrombin (PT) and activated partial thromboplastin time (PTT) are frequently prolonged due to disseminated intravascular coagulopathy (DIC) [14, 43].
Diagnosis
Initial evaluation of such an infant includes a careful physical examination, laboratory evaluation and plain abdominal radiographs [1, 3, 45].
NB: Although various clinical and radiographic signs and symptoms are used to make the diagnosis, the classic clinical triad consists of abdominal distension, bloody stools, and pneumatosis intestinalis.
On a flat anteroposterior view, bowel distension is the earliest and most common radiographic finding of NEC.
Intramural bowel gas (pneumatosis intestinalis) occurs early in the disease process in almost all patients with NEC and it is considered pathognomonic. However, pneumatosis intestinalis is not a specific finding and has been reported in several other diseases, including Hirschsprung's disease with enterocolitis, pyloric stenosis and carbohydrate intolerance. The pneumatosis may be a combination of linear (subserosal gas) or cystic (submucosal gas).
Other plain film findings include portal venous gas (10%), pneumoperitoneum (10-20%), ascites (10%), or fixed and persistently dilated bowel loops.
Only 63% of infants with intestinal perforations due to NEC demonstrate pneumoperitoneum on preoperative abdominal films. Contrast studies are rarely performed to confirm the diagnosis [14].
Ultrasonography can be used to identify portal vein gas, pneumatosis and ascites [1, 3, 8].
Some premature infants may present with pneumoperitoneum without any of the other cardinal symptoms associated with NEC. These spontaneous intestinal perforations have been associated with use of indomethacin, maternal drug use, prematurity and infection. Unlike infants with NEC, these patients have a single isolated intestinal perforation, usually ileal, the remaining bowel being normal [43].
Treatment
As is true for most diseases, prevention is the best treatment. Steps to be taken consist of [16, 36, 43]:
1. Prenatal care to decrease the incidence of premature births
2. Use of breast milk in the neonatal intensive care unit
3. Strict adherence to handwashing and isolation protocols
4. Some possible benefit to advancing the feeding schedule slowly
Initial therapy is nonoperative and is instituted immediately upon suspicion of the diagnosis of NEC.
Medical management of NEC [14]:
* Cultures: Blood cultures are necessary.
Urine/sputum/cerebrospinal fluid as indicated.
* Orogastric or nasogastric decompression of stomach.
* Intravenous fluid resuscitation to restore tissue perfusion and renal function.
* Antibiotics: Synergistic coverage for Gram-positives and Gram-negatives, with additional coverage for anaerobes.
* Correction of Anemia and Coagulopathy: Transfuse packed red blood cells and platelets. Fresh frozen plasma is used as coagulation parameters indicate.
* Abdominal supine and gravity-dependent (cross-table or left lateral decubitus) radiographs, repeated serially as clinical picture indicates.
* Frequent repeat abdominal examinations by the same physician at least every 6 hours until infant stable, then as clinical picture indicates.
* Surgical intervention if the infant worsens, fails to improve on intensive non-surgical therapy, or for advanced NEC with perforation.
Coverage for anaerobes with metronidazole or clindamycin is usually unnecessary but this decision is usually made based on institutional preferences [43].
Surgical therapy
The timing of operative treatment is based on experience and good judgment and as such is often somewhat subjective [43].
Ideally, surgery should not be undertaken until gangrene is present but be performed before perforation occurs. Unfortunately, no combination of clinical examination or adjunct testing has been shown to have high sensitivity for intestinal gangrene. Thus, there remains controversy regarding the indications for surgery, the most appropriate timing of intervention, and the optimal surgical treatment strategy [14, 45].
v "Best" indicators (specificity and positive predictive value (PPV) approaching 100%, prevalence >10%) were pneumoperitoneum, positive paracentesis (aspiration of >0,5 mL brown or yellow fluid containing bacteria on Gram stain), and portal venous gas.
v "Good" indicators (specificity and PPV approaching 100%, prevalence <10%) were a fixed intestinal loop, erythema of the abdominal wall, and a palpable abdominal mass.
v "Fair" indicator (specificity of 91%, PPV of 94%, prevalence of 20%) was "severe" pneumatosis intestinalis as graded by a radiographic system.
v "Poorer" indicators were clinical deterioration (PPV of 78%), platelet count lower than 100,000/mm3 (PPV of 50%), abdominal tenderness (PPV of 58%), severe GI hemorrhage (PPV of 50%), and gasless abdomen with ascites (0%).
Generally, the combination of persistent or worsening thrombocytopenia or acidosis despite adequate resuscitation, especially in the presence of a fixed loop (which is likely severely ischemic) on X-ray, would prompt most pediatric surgeons to operate (Table 5.7).
Table 5.7
Indications for operation in NEC [36]
|
Absolute indications |
|
|
Relative indications |
|
The main surgical options for an infant with NEC include [14]:
1. peritoneal drainage
2. laparotomy with resection and stoma(s)
3. laparotomy with resection and primary anastamosis
4. laparotomy with proximal diversion
5. a combination of 1-4
Surgical options in infants with NEC depend upon their clinical stability and the extent of disease. On exploration, if there is an isolated segment of intestinal necrosis, resection with primary anastomosis or with stomal diversion are possible options.
Laparotomy is usually carried out through a right-sided, transverse supraumbilical incision, which allows careful examination of the entire intestine.
Regardless of the procedure chosen, great care is taken to minimize evaporative heat and water losses during surgery (i.e., warming pads, plastic coverings, warmed fluids and efforts to keep the intestine inside the abdominal cavity whenever possible) [7, 36, 45].
In cases of significant bowel necrosis but with greater than 50% bowel being viable, resection with proximal stoma is a choice.
Patients with pan-necrosis present a particularly difficult problem. Multiple limited resections and/or proximal stomas can both be considered. These patients may require a second look within the next 24 hours to assess bowel viability. These infants are most at risk for developing short gut syndrome. The length of remaining viable intestine, its location and the presence or absence of the ileocecal valve are important considerations and should carefully be noted in the medical record.
In cases of severe pan-necrosis, the surgeon, in consultation with the family, may choose to explore the abdomen and close with no further surgical intervention. In this scenario, surgery is performed to confirm the diagnosis and to allow the provision of comfort care to infants with no chance of survival [14, 43].
Peritoneal drainage is often performed in infants weighing less than 1000 g and in some larger infants who are physiologically unstable. A drain (e.g., Penrose) is placed into the peritoneal cavity usually via a right lower quadrant incision. Ideally, primary abdominal exploration is the better choice; however, peritoneal drainage is a useful procedure as part of resuscitation [14, 16].
Approximately one-third of infants with NEC weighing less than 1000 g who are treated with peritoneal drainage require no further surgical intervention. On the other hand, exploration is indicated if there is no improvement within 24 hours of drain placement [14, 36, 43].
NB: Thus it is apparent that the procedure must be individualized for each patient based on size, severity of illness and presence of other comorbid factors (i.e., intraventricular hemorrhage (IVH), etc.).
After an infant recovers from an NEC episode, enteral feeding is introduced slowly after about 10-14 days of medical therapy. Enteral feeds are slowly advanced toward goal rates and concentration while nutrition is supplemented by intravenous hyper-alimentation as needed.
After recovery from NEC, 11-30% of infants develop intestinal strictures resulting from circumferential scarring of nonperforated intestinal segments. These strictures are most commonly seen in the colon (70%), usually at the splenic flexure and terminal ileum (15%). Infants with strictures become distended and enteral feeds cannot be advanced. Strictures are usually treated by surgical resection and primary anastomosis. Balloon dilation is also being tried [14].
Infants with stomas are allowed to feed and grow before the stomas are closed. An arbitrary time or size such as 2 months or 5 kg is sometimes used as a goal [14, 36]. Intestinal continuity is restored earlier if:
¤ the intestinal segment proximal to the stoma is short and is causing failure to thrive or difficult water and/or salt loss problems
¤ stomal complications occur (i.e., stenosis, prolapse)
Patients with resections leaving insufficient absorptive intestinal length have short bowel syndrome (SBS) and require long-term total parenteral nutrition (TPN).
In some of these infants, SBS is a temporary problem that resolves as intestinal length and diameter increase with age, but central venous catheter infections and complications (i.e., cholestatic liver disease, etc.) are serious, life-threatening problems that have to be kept in check.
Outcomes
Overall survival from NEC is improving; especially in those infants weighing less than 1000 g [16, 36].
Infants who progress to intestinal perforations have nearly 46-65% perioperative mortality, whereas infants without perforation at the time of surgery have 30% mortality. Survival rates for surgically treated infants with NEC are better than for those who were treated only with peritoneal drainage, except in very low birth weight infants (<1000 g) and infants with spontaneous intestinal perforation [14].
Recurrent NEC occurs in about 6% of infants and typically occurs 3-5 weeks after the first episode.
Primary peritonitis
Primary peritonitis (PP) (also referred to as idiopathic or spontaneous peritonitis) has been defined as an infectious process involving the peritoneal cavity that has no intraabdominal source.
The infection may reach the abdomen through hematogenous or lymphatic routes or by direct extension from the vagina.
With the exception of the ends of the fallopian tubes, the peritoneal cavity is a completely closed space that can be penetrated by foreign bodies such as ventriculoperitoneal shunts and peritoneal dialysis catheters. In prepubertal and adolescent girls, retrograde spread of fluid out through the fallopian tubes may account for the presence of an ascending vulvovaginitis or “swimming pool” peritonitis [14, 43].
Today, this condition represents less than 1% of all pediatric laparotomies because the diagnosis is made and treatment initiated without the need for an operation [14].
Most cases of PP in the pediatric age group are associated with nephrotic syndrome or chronic hepatic states in which ascites or cirrhosis is present. This group includes infants and children with biliary atresia, cystic fibrosis, hepatic fibrosis, and lupus erythematosus.
Conditions Associated with Primary Peritonitis in Childhood:
· Nephrotic syndrome
· Hepatic dysfunction
· Adrenogenital syndrome
· Cystic fibrosis
· Chronic renal failure with the need for chronic ambulatory peritoneal dialysis
· Complications after splenectomy
· Diseases requiring long-term steroid administration (systemic lupus erythematosus, dermatomyositis)
Organisms isolated from the peritoneal cavity will vary according to the various associated conditions.
Microorganisms Associated with Primary Peritonitis:
- Streptococcus species (S. pneumoniae; group A streptococci)
- Escherichia coli
- Gonococcus
- Haemophilus influenzae
- Klebsiella pneumoniae
- Listeria monocytogenes
- Parainfluenza
- Salmonella typhi
- Serratia marcescens
- Yersinia enterocolitica
For instance, gram-positive organisms, including Streptococcus pneumoniae and group A streptococci, and a variety of gram-negative species are most commonly found in patients with nephrotic syndrome. The same gram-positive groups are cultured in patients with underlying liver disease, whereas the spectrum of gram-negative isolates includes Esherichia coli, Klebsiella pneumoniae, and Pseudomonas species [16, 36].
In earlier reported series, most of the patients were girls and the vagina was often implicated as the source of the infections. However, vaginitis was uncommon, and the ultimate source of the infection remained obscure [14].
The prepubertal cervix lacks the endocervical glands that may harbor bacteria, so ascending infections in this age group would more likely be associated with some traumatic force that pushes the bacteria up through the vagina, as in sexual abuse cases or by jumping feet first into swimming pool or lake water.
In more recent series the incidence of PP is equally distributed between boys and girls [16, 36, 43]. In some cases the same bacteria causing the peritoneal infection have also been cultured from the respiratory and urinary tract, as well as from the oral cavity [36].
Diagnosis
Children with PP have acute abdominal pain associated with a febrile illness, nausea, vomiting, diarrhea, or other viral-like prodromes [2, 8].
The time course may be more protracted than that for secondary peritonitis, and diffuse rebound tenderness is often present. The absence of localized pain may result from irritation of visceral organ surfaces.
Samples of blood and urine are sent for culture, and radiographs of the chest and abdomen are obtained. This will help eliminate a perforated viscus or a pneumonic process as the cause of the abdominal pathology. Low serum protein levels and concomitant decreased opsonins may contribute to the development of PP [36, 45].
Ultrasound studies and CT of the abdomen have been used to differentiate primary from secondary peritonitis (caused by such common pediatric processes as appendicitis) when the clinical picture is confusing.
The diagnosis of PP is made when the peritoneal fluid leukocyte count is higher than 500/mm3 and granulocytes predominate (lymphocytes are usually present in higher numbers in normal peritoneal fluid). The fluid has a pH of less than 7.35 and an elevated lactate level (>25 mg/dL) [14, 43].
Sending a minimum of 10 mL of fluid for Gram stain and culture analysis will increase the number of positive isolates, yet cultures may be negative in as many as 60% of reported cases. If more than one organism is present on Gram stain, a perforated viscus should be suspected [14].
Treatment
Initial teatment with parenteral cephalosporins is indicated, and the presence of other associated simultaneous sexually transmitted bacteria should be investigated.
When the clinical picture is not improving despite intravenous antibiotics, diagnostic laparoscopy or laparotomy is indicated.
An appendectomy can be done safely even in the presence of cloudy exudate, and the bowel surface can be inspected for secondary causes of the infection [16, 36].
After the surgical intervention, antibiotics are continued until the leukocytosis normalizes and the ileus resolves.